Chapter: Basic & Clinical Pharmacology : Special Aspects of Perinatal & Pediatric Pharmacology
Drug Therapy in Pregnancy
DRUG THERAPY IN PREGNANCY
Pharmacokinetics
Most drugs taken by
pregnant women can cross the placenta and expose the developing embryo and
fetus to their pharmacologic and teratogenic effects. Critical factors
affecting placental drug transfer and drug effects on the fetus include the
following: (1) the physicochemical properties of the drug; (2) the rate at
which the drug
crosses the placenta and the amount of drug reaching the fetus;
the duration of exposure to the drug; (4)
distribution charac-teristics in different fetal tissues; (5) the stage of
placental and fetal development at the time of exposure to the drug; and (6)
the effects of drugs used in combination.
A. Lipid Solubility
As is true also of
other biologic membranes, drug passage across the placenta is dependent on
lipid solubility and the degree of drug ionization. Lipophilic drugs tend to
diffuse readily across the pla-centa and enter the fetal circulation. For
example, thiopental, a drug commonly used for cesarean sections, crosses the
placenta almost immediately and can produce sedation or apnea in the newborn
infant. Highly ionized drugs such as succinylcholine and tubocura-rine, also
used for cesarean sections, cross the placenta slowly and achieve very low
concentrations in the fetus. Impermeability of the placenta to polar compounds
is relative rather than absolute. If high enough maternal-fetal concentration
gradients are achieved, polar compounds cross the placenta in measurable
amounts. Salicylate, which is almost completely ionized at physiologic pH,
crosses the placenta rapidly. This occurs because the small amount of
salicylate that is not ionized is highly lipid-soluble.
B. Molecular Size and pH
The molecular weight
of the drug also influences the rate of trans-fer and the amount of drug
transferred across the placenta. Drugs with molecular weights of 250–500 can
cross the placenta easily, depending upon their lipid solubility and degree of
ionization; those with molecular weights of 500–1000 cross the placenta with
more difficulty; and those with molecular weights greater than 1000 cross very
poorly. An important clinical application of this property is the choice of
heparin as an anticoagulant in pregnant women. Because it is a very large (and
polar) molecule, heparin is unable to cross the placenta. Unlike warfarin,
which is teratogenic and should be avoided during the first trimester and even
beyond (as the brain continues to develop), heparin may be safely given to
pregnant women who need anticoagulation. Yet the placenta con-tains drug
transporters, which can carry larger molecules to the fetus. For example, a
variety of maternal antibodies cross the pla-centa and may cause fetal
morbidity, as in Rh incompatibility.
Because maternal blood
has a pH of 7.4 and that of the fetal blood is 7.3, weakly basic drugs with pKa above 7.4 will be
more ionized in the fetal compartment, leading to ion trapping and, hence, to
higher fetal levels .
C. Placental Transporters
During
the last decade, many drug transporters have been identified in the placenta,
with increasing recognition of their effects on drug transfer to the fetus. For
example, the P-glycoprotein transporter encoded by the MDR1 gene pumps back into the maternal circula-tion a variety of
drugs, including cancer drugs (eg, vinblastine, doxo-rubicin) and other agents.
Similarly, viral protease inhibitors, which are substrates of P-glycoprotein,
achieve only low fetal concentra-tions—an effect that may increase the risk of
vertical HIV infection from the mother to the fetus. The hypoglycemic drug
glyburide shows much lower concentrations in the fetus as compared to the
mother. Recent work has documented that this agent is effluxed from the fetal
circulation by the BCRP transporter as well as by the MRP3 transporter located
in the placental brush border membrane.
D. Protein Binding
The degree to which a
drug is bound to plasma proteins (particu-larly albumin) may also affect the
rate of transfer and the amount transferred. However, if a compound is very
lipid-soluble (eg, some anesthetic gases), it will not be affected greatly by protein
binding. Transfer of these more lipid-soluble drugs and their over-all rates of
equilibration are more dependent on (and proportion-ate to) placental blood
flow. This is because very lipid-soluble drugs diffuse across placental
membranes so rapidly that their overall rates of equilibration do not depend on
the free drug con-centrations becoming equal on both sides. If a drug is poorly
lipid-soluble and is ionized, its transfer is slow and will probably be impeded
by its binding to maternal plasma proteins. Differential protein binding is
also important since some drugs exhibit greater protein binding in maternal
plasma than in fetal plasma because of a lower binding affinity of fetal
proteins. This has been shown for sulfonamides, barbiturates, phenytoin, and
local anesthetic agents. In addition, very high maternal protein binding of
glyburide(>
98.8%) also contributes to lower fetal levels as compared to maternal
concentrations, as mentioned above.
E. Placental and Fetal Drug Metabolism
Two mechanisms help
protect the fetus from drugs in the maternal circulation: (1) The placenta
itself plays a role both as a semiperme-able barrier and as a site of
metabolism of some drugs passing through it. Several different types of
aromatic oxidation reactions (eg, hydroxylation, N-dealkylation, demethylation) have been shown to occur in
placental tissue. Pentobarbital is oxidized in this way. Conversely, it is
possible that the metabolic capacity of the placenta may lead to creation of
toxic metabolites, and the placenta may therefore augment toxicity (eg,
ethanol, benzpyrenes). (2) Drugs that have crossed the placenta enter the fetal
circulation via the umbilical vein. Approximately 40–60% of umbilical venous
blood flow enters the fetal liver; the remainder bypasses the liver and enters
the general fetal circulation. A drug that enters the liver may be partially
metabolized there before it enters the fetal circula-tion. In addition, a large
proportion of drug present in the umbili-cal artery (returning to the placenta)
may be shunted through the placenta back to the umbilical vein and into the
liver again. It should be noted that metabolites of some drugs may be more
active than the parent compound and may affect the fetus adversely.
Pharmacodynamics
A. Maternal Drug Actions
The
effects of drugs on the reproductive tissues (breast, uterus, etc) of the
pregnant woman are sometimes altered by the endocrine environment appropriate
for the stage of pregnancy. Drug effects on other maternal tissues (heart,
lungs, kidneys, central nervous system, etc) are not changed significantly by
pregnancy, although the physiologic context (cardiac output, renal blood flow,
etc) may be altered, requiring the use of drugs that are not needed by the same
woman when she is not pregnant. For example, cardiac glycosides and diuretics
may be needed for heart failure precipitated by the increased cardiac workload
of pregnancy, or insulin may be required for control of blood glucose in
pregnancy-induced diabetes.
B. Therapeutic Drug Actions in the Fetus
Fetal therapeutics is
an emerging area in perinatal pharmacology. This involves drug administration
to the pregnant woman with the fetus as the target of the drug. At present,
corticosteroids are used to stimulate fetal lung maturation when preterm birth
is expected. Phenobarbital, when given to pregnant women near term, can induce
fetal hepatic enzymes responsible for the glucuronidation of bilirubin, and the
incidence of jaundice is lower in newborns when mothers are given phenobarbital
than when phenobarbital is not used. Before phototherapy became the preferred
mode of therapy for neonatal indirect hyperbilirubine-mia, phenobarbital was
used for this indication. Administration of phenobarbital to the mother was
suggested recently as a means of decreasing the risk of intracranial bleeding
in preterm infants. However, large randomized studies failed to confirm this
effect. Antiarrhythmic drugs have also been given to mothers for treatment of
fetal cardiac arrhythmias. Although their efficacy has not yetbeen established
by controlled studies, digoxin, flecainide, pro-cainamide,
verapamil, and other antiarrhythmic agents have been shown to be effective in
case series. During the last two decades it has been shown that maternal use of
zidovudine decreases by two thirds transmission of HIV from the mother to the
fetus, and use of combinations of three antiretroviral agents can eliminate
fetal infection almost entirely .
C. Predictable Toxic Drug Actions in the Fetus
Chronic use of opioids
by the mother may produce dependence in the fetus and newborn. This dependence
may be manifested after delivery as a neonatal withdrawal syndrome. A less well
under-stood fetal drug toxicity is caused by the use of angiotensin-converting
enzyme inhibitors during pregnancy. These drugs can result in significant and
irreversible renal damage in the fetus and are therefore contraindicated in
pregnant women. Adverse effects may also be delayed, as in the case of female
fetuses exposed to diethylstilbestrol, who may be at increased risk for
adenocarci-noma of the vagina after puberty.
D. Teratogenic Drug Actions
A single intrauterine
exposure to a drug can affect the fetal struc-tures undergoing rapid
development at the time of exposure.Thalidomide is an example of a drug that
may profoundly affect the development of the limbs after only brief exposure.
This expo-sure, however, must be at a critical time in the development of the
limbs. The thalidomide phocomelia risk occurs during the fourth through the
seventh weeks of gestation because it is during this time that the arms and
legs develop (Figure 59–1).
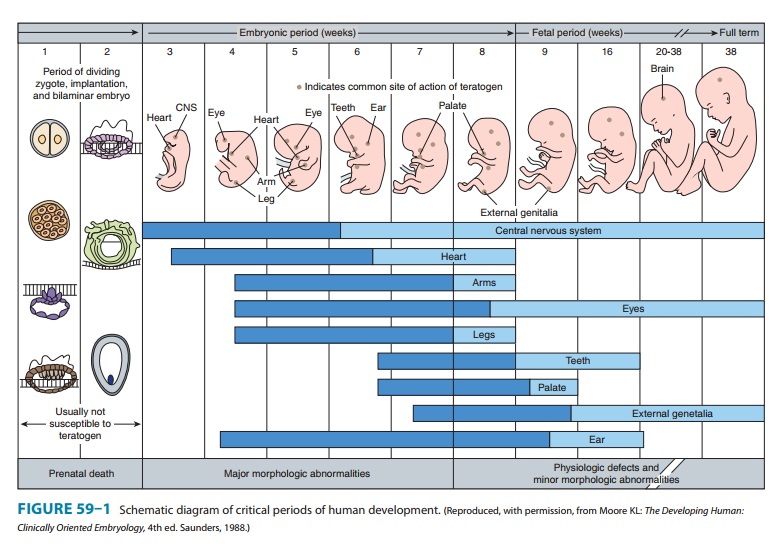
1. Teratogenic mechanisms—The mechanisms by whichdifferent drugs produce teratogenic effects are poorly understood and are probably multifactorial. For example, drugs may have a direct effect on maternal tissues with secondary or indirect effects on fetal tissues. Drugs may interfere with the passage of oxygen or nutrients through the placenta and therefore have effects on the most rapidly metabolizing tissues of the fetus. Finally, drugs may have important direct actions on the processes of differentia-tion in developing tissues. For example, vitamin A (retinol) has been shown to have important differentiation-directing actions in normal tissues. Several vitamin A analogs (isotretinoin, etretinate) are powerful teratogens, suggesting that they alter the normal processes of differentiation. Finally, deficiency of a critical sub-stance appears to play a role in some types of abnormalities. For example, folic acid supplementation during pregnancy appears to reduce the incidence of neural tube defects (eg, spina bifida).
Continued
exposure to a teratogen may produce cumulative effects or may affect several
organs going through varying stages of development. Chronic consumption of high
doses of ethanol dur-ing pregnancy, particularly during the first and second
trimesters, may result in the fetal alcohol syndrome . In this syndrome, the
central nervous system, growth, and facial develop-ment may be affected.
2. Defining a teratogen—To
be considered teratogenic, acandidate substance or process should (1) result in
a characteristic set of malformations, indicating selectivity for certain
target organs; (2) exert its effects at a particular stage of fetal
develop-ment, eg, during the limited time period of organogenesis of the target
organs (Figure 59–1); and (3) show a dose-dependent inci-dence. Some drugs with
known teratogenic or other adverse effects in pregnancy are listed in Table
59–1. Teratogenic effects are not limited only to major malformations, but also
include intrauterine growth restriction (eg, cigarette smoking), miscarriage
(eg, alcohol), stillbirth (eg, cigarette smoke), and neurocognitive delay (eg,
alcohol).
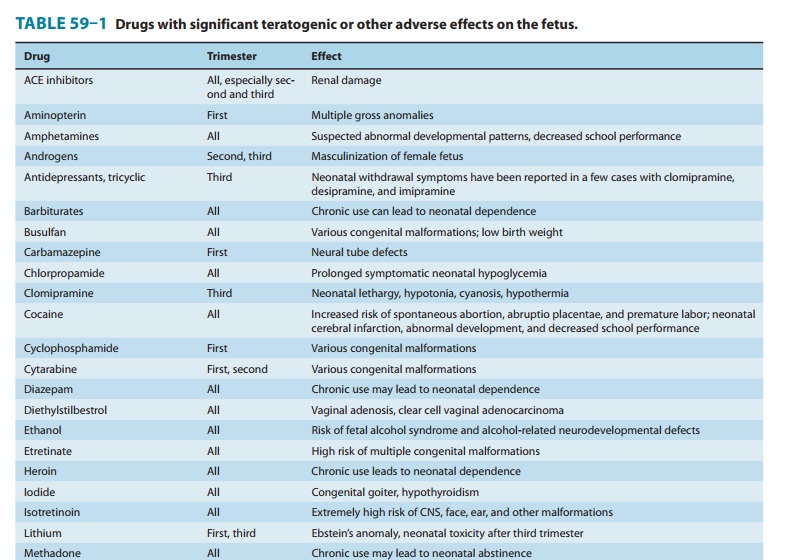
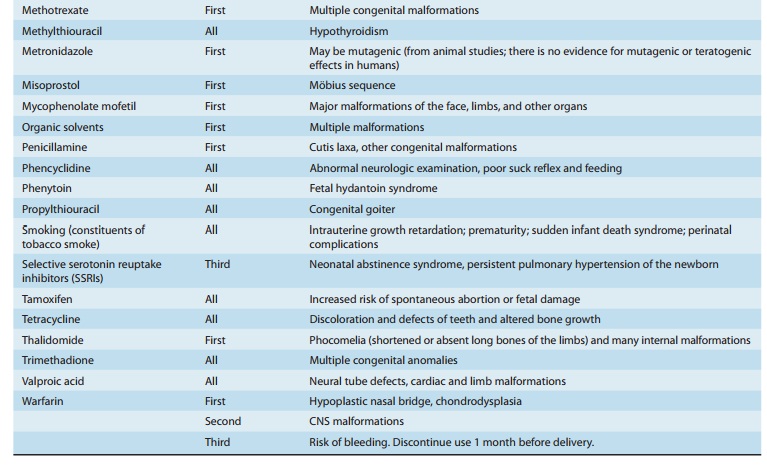
The widely cited Food
and Drug Administration (FDA) sys-tem for teratogenic potential (Table 59–2) is
an attempt to quan-tify teratogenic risk from A (safe) to X (definite human
teratogenic risk). This system has been criticized as inaccurate and
impracti-cal. For example, several drugs have been labeled “X” despite
extensive opposite human safety data (eg, oral contraceptives). Diazepam and
other benzodiazepines are labeled as “D” despite lack of positive evidence of
human fetal risk. Presently the FDA is changing its system from the A, B, C
grading system to narrative statements that will summarize evidence-based
knowledge about each drug in terms of fetal risk and safety.
3. Counseling
women about teratogenic risk—Since thethalidomide disaster, medicine has been practiced as if
every drug were a potential human teratogen when, in fact, fewer than 30 such
drugs have been identified, with hundreds of agents proved safe for the unborn.
Owing to high levels of anxiety among preg-nant women—and because half of the
pregnancies in North America are unplanned—every year many thousands of women
need counseling about fetal exposure to drugs, chemicals, and
In the
Motherisk program in Toronto, thousands of women are counseled every month, and
the ability of appropriate counseling to prevent unnecessary abortions has been
docu-mented. Clinicians who wish to provide such counsel to pregnant women must
ensure that their information is up-to-date and evidence-based and that the
woman understands that the baseline teratogenic risk in pregnancy (ie, the risk
of a neonatal abnormal-ity in the absence of any known teratogenic exposure) is
about 3%. It is also critical to address the maternal-fetal risks of the
untreated condition if a medication is avoided. Recent studies show serious
morbidity in women who discontinued selective serotonin reuptake inhibitor
therapy for depression in pregnancy.
Related Topics