Chapter: Clinical Cases in Anesthesia : Liver Disease
Describe the basic hepatic functions that are of immediate concern to anesthesiologists
Describe
the basic hepatic functions that are of immediate concern to anesthesiologists.
Because of the myriad of liver functions that
may be affected, no single laboratory test effectively measures the overall
state of liver function. An understanding of hepatic functions and coexisting
physiologic problems associated with liver failure will illuminate where the
anesthetic concerns are and which preoperative test should be performed. In any
given patient with hepatic disease, their ability to carry out normal hepatic
functions will be char-acterized by the extent of liver failure. It is
auspicious that the liver has an enormous reserve capacity. In experimental
animal models, as well as in normal humans, the removal of greater than 80% of
hepatic parenchyma is still compat-ible with normal liver function. Cirrhosis is
characterized as scarring within the liver by fibrosis and the conversion of
normal architecture into structurally abnormal modules throughout the liver.
This abnormal architecture leads to obstruction of flow within the portal
system and portal hypertension with all its clinical ramifications. The
ulti-mate consequence of progressive liver diseases is hepatic failure and loss
of liver function. Hepatic functions can be broken down into three main
categories: endocrine, synthetic (anabolic), and metabolic (catabolic and
detoxi-fying) functions.
Endocrine Functions
The liver has several endocrine functions and
is a major target organ for glucose homeostasis. The liver produces somatomedin
(insulin-like growth factor-1), a growth stimulator; thrombopoietin, which
stimulates bone mar-row to produce platelets; and angiotensinogen, which is
closely involved in fluid and electrolyte balance.
Decreased production of angiotensinogen can
have profound effects on the kidneys and fluid and electrolyte balance. Extravasation
of fluid from the intravascular volume results in relative intravascular
depletion. The kid-ney responds by producing increased amounts of renin. Two
physiologic ramifications of increased renin produc-tion are constriction of
the renal afferent arteries and fluid retention. Renin also converts
angiotensinogen into angiotensin I. Angiotensin I diminishes renin production
by the kidneys. Without production of angiotensinogen, the negative feedback
that inhibits renin production is eliminated, and production of renin and its
physiologic ramifications go unconstrained. This cycle can cause massive fluid
retention, electrolyte abnormalities, and may play a role in the development of
hepatorenal syndrome in end-stage liver disease (ESLD).
The liver plays a role in calcium homeostasis.
It is respon-sible for the hydroxylation of vitamin D. Additionally, the liver
is responsible for the homeostasis of other hormones. Thyroxine and
triiodothyronine are deiodinated by the liver. Steroid hormones, such as testosterone,
estradiol, glucocor-ticoids, and aldosterone, are first metabolized
(inactivated) and conjugated in the liver and then excreted in the urine. In
liver disease, normal estrogen and testosterone metabolism is prevented because
of shunting to the systemic circulation, which results in gynecomastia.
Furthermore, the liver is a target organ for
insulin and glucagon. These two hormones are involved in the metabolism and
storage of carbohydrates. Glycogen is formed from glucose, under the influence
of insulin, in a process called glycogenesis. Glycogen is broken down to
glucose by glucagon in a process called glycogenolysis.
In this way, glucose becomes available for
muscle and brain metabolism.
Anabolic Functions
The liver has numerous clinically important
synthetic functions. It is involved in hemostasis by virtue of its anabolic
functions. In addition to producing coagulation factors, the liver also
synthesizes many anticoagulants, such as antithrombin III, α1-antitrypsin,
protein C and S, plas-minogen, α2-antiplasmin, and plasminogen activator inhibitor. Severe liver
disease can lead to reduced synthesis of factors I (fibrinogen), II
(prothrombin), V, VII, IX, X, XI, XII, XIII, prekallikrein, and high molecular
weight kinino-gen. High molecular weight kininogen and factors II, VII, IX, and
X are vitamin-K-dependent clotting factors. Vitamin K is a cofactor of an
enzyme that catalyzes the Îł-carboxylation of selected glutamyl residues in
clotting factor precursors. When coagulopathies result from impaired
hepatocellular function, exogenous vitamin K is unlikely to correct or improve
the problem. Vitamin K-dependent clotting proteins have a substantially shorter
serum half-life than albumin; therefore, coagulopathy can precede the
development of other signs of liver failure, e.g., hypoalbuminemia. In
cirrhosis, coagulopathy may be further aggravated by thrombocytopenia resulting
from either decreased synthesis of thrombopoietin and/or from hypersplenism.
Since the liver produces non-vitamin K-dependent clotting factors, severe liver
disease may lead to decreased plasma concentrations of factor I (fibrinogen),
V, XI, XII, and XIII. Initially, a damaged liver may actually produce increased
amounts of fibrinogen; therefore, it is unusual for fibrinogen to be reduced
significantly, unless there is an associated disseminated intravascular
coagulation.
Several other clinically important proteins are
made by the liver. They include acute phase reactants (C-reactive proteins,
haptoglobin, ceruloplasmin, and transferrin), pseudocholinesterase,
angiotensinogen (discussed above), α-acid glycoprotein, and albumin. The last two
are the main drug-binding moieties. Derangements in albumin synthesis have
several important clinical ramifications. It is the principal binding and
transport protein for numerous substances, including some hormones, fatty
acids, trace metals, bilirubin, and drugs. Many of the intravenous drugs
employed by anesthesiologists are highly protein-bound. Low serum
concentrations of plasma proteins, especially albumin, produce an increase in
unbound drug concentrations and potentially exaggerate drug responses. This
enhanced response may be seen with serum albumin concentrations of 2.5 g/dL or
less. Additionally, decreased serum concentrations will lead to reduced oncotic
pressure in the plasma, which may result in edema and ascites. When this is
coupled with portal hypertension, there may be increased hepatic lymph
production with extravasation into the peritoneal cavity. In a patient with
ascites, the degree and ramifications of hypoalbuminemia may be accentuated by
further loss of albumin in ascitic fluid.
Other important anabolic functions of the liver
include production of saturated fatty acids, cholesterol, and bile salts,
maintenance of glycogen storage (glucose storage), and production of ketones,
i.e., β-hydroxybutyrate, which is the main energy source used by the brain
during starva-tion. Decreased synthesis of bile salts can lead to
malab-sorption of fat and fat-soluble vitamins (vitamin K). Alterations in
cholesterol production (and related sub-stances) may lead to significant
changes in the composition and morphology of erythrocytes. The presence of
erythro-cytes with spur and burr cell forms are usually an ominous sign of
significantly advanced liver disease.
Abnormalities of glucose maintenance are common
in cirrhosis. As stated above, carbohydrate metabolism and glucose production
are important liver functions. Hypoglycemia, which is more commonly associated
with acute fulminant hepatic failure, may occur with ESLD. Cirrhotic patients
are at risk for perioperative hypoglycemia due to decreased hepatic glycogen
stores (decreased capacity secondary to decreased liver mass or decreased
intrinsic ability to synthesize glycogen), diminished response to glucagon, or
compromised nutritional status. Additionally, these patients may have elevated
serum lactate levels, reflecting the decreased capacity of the liver to utilize
lactate for gluconeogenesis via the Cori cycle (Figure 35.1).
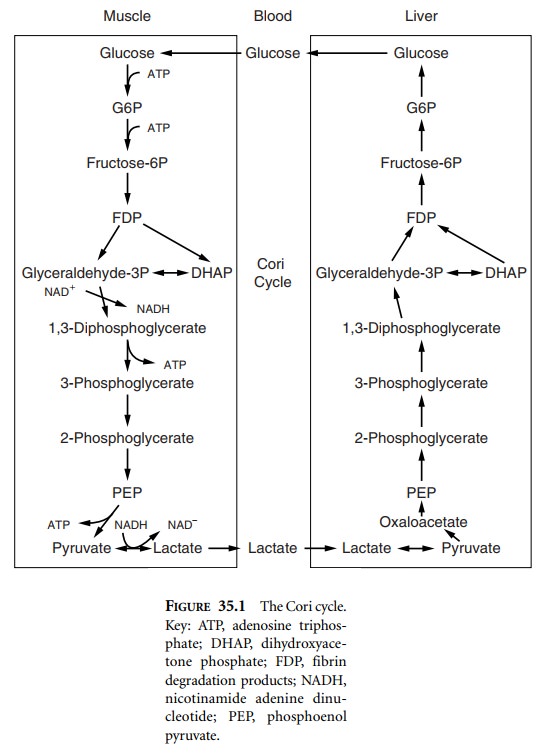
Catabolic Functions
The liver is responsible not only for
elimination and metabolism of toxins and other xenobiotics absorbed by the
gastrointestinal tract, but also for metabolism of drugs and alcohol.
The liver is the central organ responsible for
biochemi-cal intermediate metabolism. It shuffles many endogenous biological
intermediate compounds into various pathways that lead to either the creation
of new compounds or the complete metabolism of the intermediate ones. A perfect
example of intermediate metabolism is the citrate pathway (Figure 35.2).
Citrate is used as an anticoagulant in banked
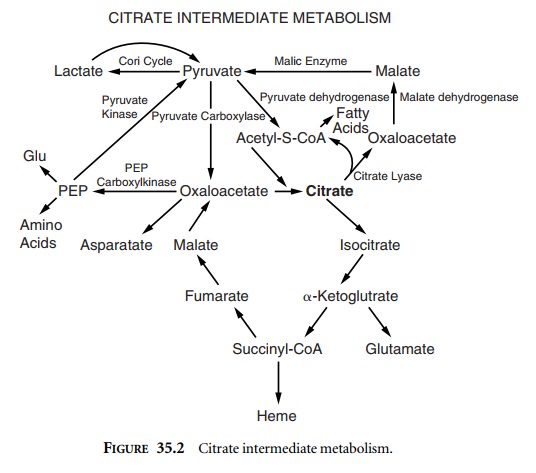
Citrate works as an
anticoagulant via chelation of calcium, thereby blocking its availability for
the coagulation cascade. Exogenously administered citrate, such as that from
fresh frozen plasma or other blood prod-ucts, is mainly metabolized by the
liver. Citrate toxicity can occur when blood products are transfused at a rapid
rate, or when the liver is unable to metabolize citrate appropri-ately.
Toxicity results from chelation of ionized calcium by citrate. As citrate
accumulates, ionized calcium levels decrease, resulting in a coagulopathy and
myocardial depression leading to hypotension.
Carbohydrate and other biological intermediate
metab-olism is a vital function of the liver. The liver metabolizes glucose,
fructose, lactate, citrate, acetate, and other biolog-ical intermediates. As
function declines, the liver loses its ability to orchestrate intermediate
metabolism. Frequently, cirrhotics may develop insulin resistance and
consequently hyperglycemia and glucose intolerance. The hyperinsulin-emia
associated with ESLD suggests a decrease in the liver’s intrinsic ability to
handle a glucose load secondary to a decrease in hepatocellular function and/or
mass. Lactic acid is produced peripherally but is metabolized in the liver.
Elevated serum lactate levels may reflect the decreased capacity of the liver
to utilize lactate and may result in metabolic acidosis (Figure 35.1).
The liver is responsible for amino acid
degradation (and production of glucose), fatty acid metabolism (β-oxidation), and the production of ketones during prolonged
fasting.
The toxic byproduct of amino acid degradation
is ammo-nia (NH4+). Disposal of ammonia via the
production of urea is an important liver function.
Related Topics