Chapter: Essential Clinical Immunology: Immune Regulation
Cytokine Immunomodulation
CYTOKINE IMMUNOMODULATION
Although many of the immunoregulation techniques
revolve around the humoral arm of the immune response, there is increasing
interest in targeting T cells or the cytokine regulators to regulate the
elimination of autoreactive T cells, tumor cells, and the maintenance of a
specific memory response to these pathogens. Such immune responses are normally
regulated by cytokines, and the activities of the cyto-kines have a high degree
of redundancy. The common cytokine receptor γ chain is used by IL-2, IL-4,
IL-7, IL-9, IL-15, and IL-21 (see Figure 3.2). The IL-2R and the IL-15R have a
second (β chain)
subunit in common as well, but have unique α sub-units (see Figure 3.3).
These two cytokine receptors use a common signaling path-way that involves
JAK1, JAK3, and STAT5. The signaling involves the phosphoryla-tion of STAT5
that results in the dissocia-tion of STAT5 from the receptor and the subsequent
dimerization of STAT5. The dimers then translocate to the nucleus and promote
transcription of target genes (see Figure 3.2).
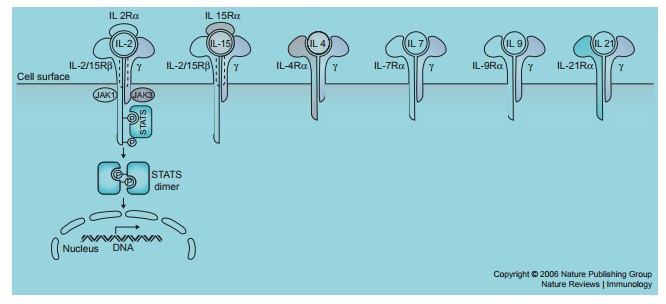
Figure 3.2 The structure and signaling pathways of the common cytokine-receptor γ chain family of receptors. Reprinted by permission from Macmillan Publishers: Waldmann T. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595
There are
several distinct differences in the functions of IL-2 and IL-15. IL-2 is
involved in checkpoints or brakes on the immune system. IL-2 is required to
maintain the competitiveness of forkhead box P3- (FoxP3-) expressing regulatory
T cells, and plays a crucial role in activa-tion-induced cell death (AICD), a
process that leads to the elimination of self-reactive T cells. Thus, the
unique feature of IL-2 is to prevent T-cell immune response to self that would
lead to autoimmunity. In contrast, IL-15 expression has no effect on regulatory
T cells but is an anti-apoptotic factor in several systems. Furthermore, IL-15
promotes the maintenance of CD8+ CD44 memory T cells. Thus, IL-15
has as its primary role the maintenance of a robust memory response to invading
pathogens.
These
observations from ex vivo func-tional studies are supported by studies in mice
deficient in cytokine or cytokine recep-tor genes. For example, IL-2-deficient
and IL-2Rα-deficient
mice develop a marked
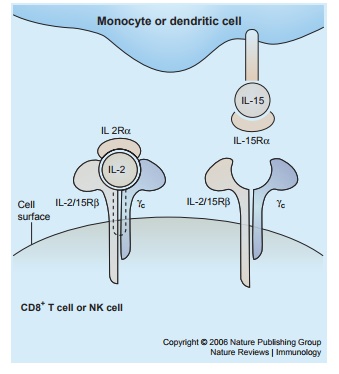
Figure 3.3 The
mode of interaction of IL-2 and
IL-15 with the subunits of their receptors. Reprinted by permission from
Macmillan Publishers: Waldmann T. The biology of interleukin-2 and
interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595.
enlargement
of the peripheral lymphoid organs and polyclonal expansion of T and B cells, and
this proliferation reflects the impairment of regulatory T cells and AICD.
These mice develop autoimmune diseases such as hemolytic anemia and
inflamma-tory bowel disease.
In
contrast, mice that are deficient in IL-15, or its receptor IL-15Rα, do not develop enlarged
lymphoid tissues, increased serum immunoglobulin, or autoimmune disease. Rather
they have a marked reduc-tion in the number of thymic and periph-eral natural
killer (NK) cells, NK T cells, and intestinal intraepithelial lympho-cytes. Thus,
IL-15Rα-deficient
mice show a marked reduction in CD8+CD44hi mem-ory T
cells. The differences in the func-tions of IL-2 and IL-15 reflect the distinct
modes of actions of these two cytokines.
IL-2
functions as a secreted cytokine, act-ing on preformed heterotrimeric receptors
expressed on activated T and NK cells (Figure 3.3). In contrast, IL-15 acts as
part of an immunological synapse. IL-15 and IL-15Rα expressed on the surface of
anti-gen-presenting cells are presented in trans to CD8 T cells and NK cells
that express only the β and γ chain of the IL-15 receptor
(Figures 3.3 and 3.4).
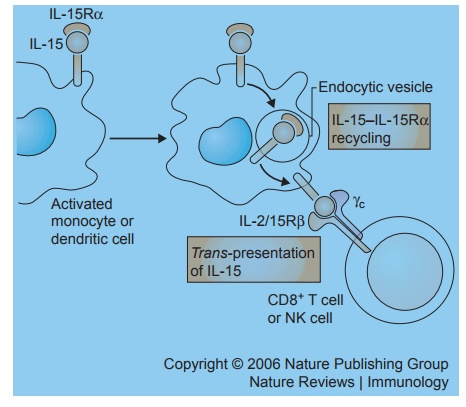
Figure 3.4 Following their co-induction on antigen-presenting cells by addition of an interferon, the interleukin receptor α chain presents IL-15 to neighboring natural killer cells and memory phenotype CD8 T cells that express the other IL-15 receptors beta and gamma. Reprinted by permission from Macmillan Publishers: Waldmann T. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595.
The
unique IL-2 and IL-2Rα system
has been a valuable target for immunotherapy because IL-2Rα is not expressed by any resting
cells with the exception of T regu-latory cells. Yet, it is expressed by many
malignant cells of various T- and B-cell leukemias, T cells that participate in
organ-allograft rejection, and finally by T cells involved in autoimmune
diseases. Thus, antibodies directed against IL-2Rα (sev-eral such antibodies have
been approved by FDA for use in humans) specifically destroy those cells that
are IL-2 dependent. One of these antibodies (daclizumab by Hoffmann – La Roche)
is in phase II trials in patients with uveitis, in certain multiple sclerosis
patients, and in asthma patients. Finally, it was shown to be effective in a
subset of patients with adult T-cell leukemia due to human T-cell lymphotropic
virus I.
It is believed that IL-15 might contrib-ute to autoimmune diseases by inducing the expression of TNF-α, by inhibiting self-tolerance mediated by IL-2-induced AICD, and by facilitating the maintenance of CD8+ memory T-cell survival, includ-ing that of self-reactive memory cells. For example, dysregulated IL-15 expression has been reported in patients with a range of autoimmune diseases such as multiple sclerosis, inflammatory bowel disease, and psoriasis. In this context, cytokine-directed blockade of TNF with specific monoclonal antibodies or soluble TNF receptors has been an important target for many of the diseases mentioned previously. Yet TNF-directed therapies do not provide effective therapy for all these patients, and new therapeutic targets are needed. Furthermore, although TNF-directed therapy has an anti-inflammatory effect, it does not have an effect on self-reactive memory T cells that might play a role in the pathogenesis and maintenance of auto-immune diseases. Thus, it is hoped that by targeting IL-15 it might be possible to both achieve the anti-inflammatory effects and reduce the number of CD8+ self-reactive memory T cells.
Several agents that inhibit IL-15 activ-ity have
been developed, including sol-uble IL-15Rα, mutant IL-15 molecules, and antibodies specific for IL-2/IL-15Rβ.
For example, in vivo, the IL-15 mutant markedly
diminished antigen-specific delayed-type hypersensitivity responses in Balb/c
mice and increased survival of pancreatic islet cell allografts. The use of
soluble high-affinity IL-15Rα
inhibited the development of mouse collagen-induced arthritis and inhibited
allograft rejection. An antibody specific for IL-15 has been efficacious in
mouse models of psoriasis. This antibody is now in a phase I/II clinical trial
in patients with rheumatoid arthritis. Finally, a humanized antibody specific
for IL-2/IL-15Rβ when administered as a single
agent prolonged cardiac-allograft survival in cynomolgus monkeys, and only
minimal toxicity was noted when this antibody was given in a phase I trial to
patients with T-cell large granular lymphocytic leukemia.
Related Topics