Chapter: Modern Pharmacology with Clinical Applications: Metabolism and Excretion of Drugs
Conjugative Enzymes: Phase II Reactions
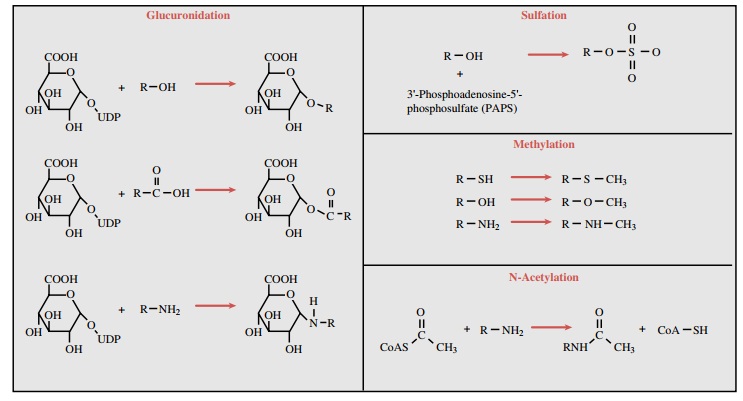
CONJUGATIVE
ENZYMES: PHASE II REACTIONS
Phase II conjugative enzymes
metabolize drugs by at-taching (conjugating) a more polar molecule to the
original drug molecule to increase water solubility, thereby permitting more
rapid drug excretion. This con-jugation can occur following a phase I reaction
involv-ing the molecule, but prior metabolism is not required. The phase II
enzymes typically consist of multiple iso-forms, analogous to the CYPs, but to
date are less well defined.
Glucuronosyl Transferases
Glucuronosyl transferases (UGTs)
conjugate the drug molecule with a glucuronic acid moiety, usually through
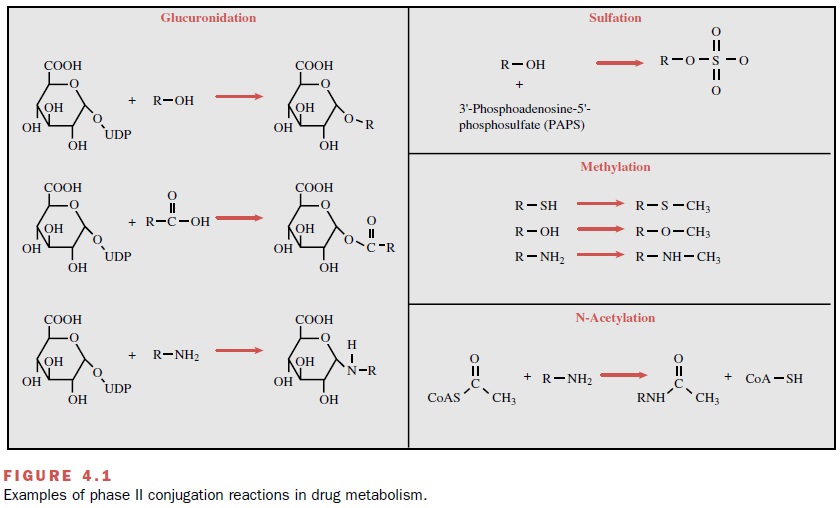
establishment of an ether, ester, or amide bond. Examples of each of these
types of conjugates are pre-sented in Figure 4.1. The glucuronic acid moiety,
being very water soluble, generally renders the new conjugate more water
soluble and thus more easily eliminated. Typically this conjugate is inactive,
but sometimes it is active. For example, UGT-mediated conjugation of morphine
at the 6- position results in the formation of morphine-6-glucuronide, which is
50 times as potent an analgesic as morphine.
It is now apparent that UGTs
are also a superfamily of enzyme isoforms, each with differing substrate
speci-ficities and regulation characteristics. Of the potential products of the
UGT1 gene family, only expression of UGT1A1, 3, 4, 5, 6, 9 and 10 occurs in
humans. Depending on the isoform, these enzymes have varying reactivity toward
a number of pharmacologically active compounds, such as opioids, androgens,
estrogens, progestins, and nonsteroidal antiinflammatory drugs; UGT1A1 is the
only physiologically significant enzyme involved in the conjugation of
bilirubin. UGT1A4 ap-pears to be inducible by phenobarbital administration, and
UGT1A7 is induced by the chemopreventive agent oltipraz.
UGT2B7 is probably the most
important of the UGT2 isoforms and possibly of all of the UGTs. It ex-hibits
broad substrate specificity encompassing a vari-ety of pharmacological agents,
including many already mentioned as substrates for the UGT1A family. Little is
known about the substrate specificities of the other UGT2B isoforms or the
inducibility of this enzyme family.
N-Acetyltransferases
As their name implies, the N-acetyltransferase (NAT) enzymes catalyze to a drug molecule the conjugation of an acetyl moiety derived from acetyl coenzyme A. Examples of this type of reaction are depicted in Figure 4.1. The net result of this conjugation is an increase in water solubility and increased elimination of the com-pound. The NATs identified to date and involved in hu-man drug metabolism include NAT-1 and NAT-2. Little overlap in substrate specificities of the two isoforms appears to exist. NAT-2 is a polymorphic enzyme, a property found to have important pharmacological con-sequences (discussed later).
To date, little
information exists on the regulation of the NAT enzymes, such as whether they
can be induced by chemicals. However, re-ports have suggested that disease
states such as ac-quired immunodeficiency syndrome (AIDS) may down-regulate
NAT-2, particularly during active dis-ease.
Sulfotransferases and Methyltransferases
Sulfotransferases (SULTs) are
important for the me-tabolism of a number of drugs, neurotransmitters, and
hormones, especially the steroid hormones. The co-substrate for these reactions
is 3 -phosphoadenosine 5 -phosphosulfate (PAPS) (Fig. 4.1). Like the
afore-mentioned enzymes, sulfate conjugation typically ren-ders the compound
inactive and more water soluble. However, this process can also result in the
activation of certain compounds, such as the antihypertensive minox-idil and
several of the steroid hormones. Seven SULT isoforms identified in humans,
including SULTs 1A1 to 1A3, possess activity toward phenolic substrates such as
dopamine, estradiol, and acetaminophen. SULT1B1 possesses activity toward such
endogenous substrates as dopamine and triiodothyronine. SULT1E1 has
substan-tial activity toward steroid hormones, especially estra-diol and
dehydroepiandrosterone, and toward the anti- hypertensive minoxidil. SULT2A1
also is active against steroid hormones. Little is known about the substrate
specificity of SULT1C1. Regulation of the SULT en-zymes appears to be
controlled by levels of the avail-able sulfate pool in the body or that of
PAPS. Patients who consume a low-sulfate diet or have ingested multi-ple SULT
substrates may be susceptible to inadequate metabolism by this enzyme and thus
drug toxicity.
The methyltransferases (MTs)
catalyze the methyl conjugation of a number of small molecules, such as drugs,
hormones, and neurotransmitters, but they are also responsible for the
methylation of such macromol-ecules as proteins, RNA, and DNA. A representative
reaction of this type is shown in Figure 4.1. Most of the MTs use S-adenosyl-L-methionine (SAM) as the
methyl donor, and this compound is now being used as a di-etary supplement for
the treatment of various condi-tions. Methylations typically occur at oxygen,
nitrogen, or sulfur atoms on a molecule. For example, catechol-O-methyltransferase (COMT) is
responsible for the bio-transformation of catecholamine neurotransmitters such
as dopamine and norepinephrine. N-methylation
is a well established pathway for the metabolism of neu-rotransmitters, such as
conversion of norepinephrine to epinephrine and methylation of nicotinamide and
hista-mine. Possibly the most clinically relevant example of MT activity
involves S-methylation by the enzyme
thiopurine methyltransferase (TPMT). Patients who are low or lacking in TPMT
(i.e., are polymorphic) are at high risk for development of severe bone marrow
sup-pression when given normal doses of the chemothera-peutic agent
6-mercaptopurine. Patients are now studied for TPMT activity prior to
administration of 6-mercap-topurine so that the dose may be adjusted downward
if they are found to be deficient in this enzyme.
Related Topics