Chapter: Organic Chemistry: Stereochemistry
Configurational isomers ŌĆō alkenes and cycloalkanes
CONFIGURATIONAL ISOMERS ŌĆō ALKENES AND
CYCLOALKANES
Key Notes
Definition
Configurational
isomers have the same molecular formula and the same bonds. However, some of
the atoms are arranged differently in space with respect to each other, and the
isomers cannot be interconverted without breaking a covalent bond. Substituted
alkenes and cycloalkanes can exist as configurational isomers.
Alkenes ŌĆō cis and trans isomerism
Alkenes
having two different substituents at each end of the double bond can exist as
two configurational isomers. Simple alkenes can be defined as cis ortransdepending on whether substituents at different ends of
thealkene are on the same side of the alkene (i.e. cis) or on opposite sides (i.e. trans).
Alkenes ŌĆō Z and E nomenclature
Alkenes
can be assigned as Z or E depending on the relative positions of
priority groups. If the priority groups at each end of the alkene are on the
same side of the double bond, the alkene is the Z isomer. If they are on opposite sides, the alkene is defined as
the E isomer. Priority groups are
determined by the atomic numbers of the atoms directly attached to the alkene.
If there is no distinction between these atoms, the next atom of each
substituent is compared.
Cycloalkanes
Substituted
cycloalkanes can exist as configurational isomers where the substituents are cis or trans with respect to each other.
Definition
Configurational isomers are isomers which have
the same molecular formula and the same molecular structure. In other words,
they have the same atoms and the same bonds. However, the isomers are different
because some of the atoms are arranged differently in space, and the isomers
cannot be interconverted without breaking and remaking covalent bonds. As a
result, configurational isomers are different compounds having different
properties.
Common examples of configurational isomers
are substituted alkenes
and substituted cycloalkanes where the substituents are
arranged differently with respect to each other.
Alkenes ŌĆō cis and transisomerism
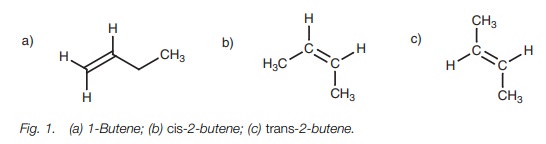
Alkenes having identical substituents at either end of the double bond can only exist as one molecule. However, alkenes having different substituents at both ends of the double bond can exist as two possible isomers. For example, 1-butene (Fig. 1a) has two hydrogens at one end of the double bond and there is only one way of constructing it. On the other hand, 2-butene has different substituents at both ends of the double bond (H and CH3) and can be constructed in two ways. The methyl groups can be on the same side of the double bond (the cis isomer; Fig. 1b), or on opposite sides (the trans isomer; Fig. 1c). The cis and trans isomers of an alkene are configurational isomers (also called geometric isomers) because they have different shapes and cannot interconvert since the double bond of an alkene cannot rotate. Therefore, the substituents are ŌĆśfixedŌĆÖ in space relative to each other. The structures are different compounds with different chemical and physical properties.
Alkenes ŌĆō Z and E nomenclature
The cis
and trans nomenclature for alkenes is
an old method of classifying the configurational isomers of alkenes and is
still commonly used. However, it is only suitable for simple 1,2-disubstituted
alkenes where one can compare the relative position of the two substituents
with respect to each other. When it comes to trisubstituted and
tetrasubstituted alkenes, a different nomenclature is required.
The Z/E nomenclature allows a clear,
unambiguous definition of the configu-ration of alkenes. The method by which
alkenes are classified as Z or E is illus-trated in Fig. 2. First of all, the atoms directly
attached to the double bond are identified and given their atomic number (Fig. 2b). The next stage is to compare
the two atoms at each end of the alkene. The one with the highest atomic number
takes priority over the other (Fig. 2c).
At the left hand side, oxygen has a higher atomic number than hydrogen and
takes priority. At the right hand side, both atoms are the same (carbon) and we
cannot choose between them.
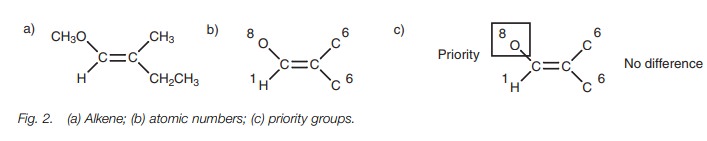
Therefore, we now need to identify the atom of highest atomic number attached to each of these identical carbons. These correspond to a hydrogen for the methyl substituent and a carbon for the ethyl substituent. These are now different and so a priority can be made (Fig. 3a). Having identified which groups have priority, we can now see whether the priority groups are on the same side of the double bond or on opposite sides. If the two priority groups are on the same side of the double bond, the alkene is designated as Z (from the German word ŌĆśzusammenŌĆÖ meaning together). If the two priority groups are on opposite sides of the double bond, the alkene is designated as E (from the German word ŌĆśentgegenŌĆÖ meaning across). In this example, the alkene is E (Fig. 3b).
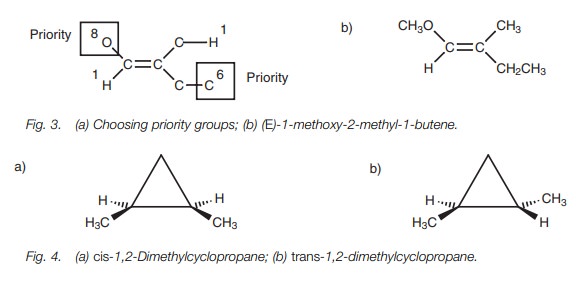
Cycloalkanes
Substituted cycloalkanes can also exist as
configurational isomers. For example, there are two configurational isomers of
1,2-dimethylcyclopropane depending on whether the methyl groups are on the same
side of the ring or on opposite sides (Fig.
4). The relative positions of the methyl groups can be defined by the
bonds. A solid wedge indicates that the methyl group is coming out the page
towards you, whereas a hatched wedge indicates that the methyl group is
pointing into the page away from you. If the substituents are on the same side
of the ring, the structure is defined as cis.
If they are on opposite sides, the structure is defined as trans.
Related Topics