Chapter: Essentials of Psychiatry: Psychiatric Pathophysiology: Addiction
Brain Circuitry and Addiction
Brain Circuitry and Addiction
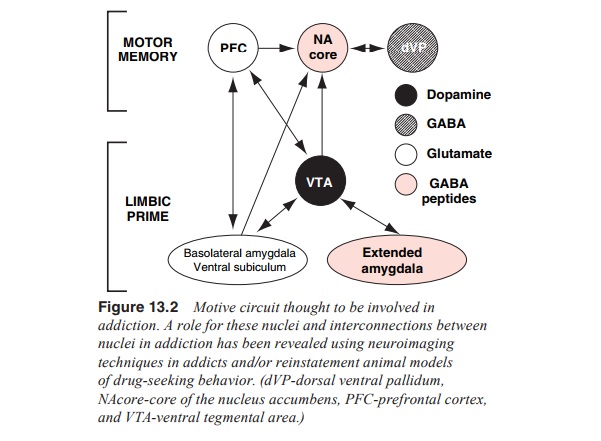
Figure 13.2 will be used as a guide and outlines
the nuclei and the interconnections between these nuclei, which will be
discussed. This circuit has been previously characterized as the motive circuit
and contains brain nuclei that are considered critical substrates for drug
reward and the devel-opment of addiction (as outlined above), such as the
dopamine neurons in the ventral tegmental area and projections to the nu-cleus
accumbens. In addition, the circuit contains prefrontal cor-tical and
allocortical brain regions now known to be critical for the expression of
behaviors commonly associated with addiction, such as drug craving.
Human Neuroimaging and Animal Models Reveal Addiction Circuitry
The majority of our recent understanding of how the
motive cir-cuit (in Figure 13.2) is involved in addiction is derived from
neu-roimaging studies in human addicts and animal studies employ-ing the
reinstatement model of drug craving. The neuroimaging studies typically involve
functional imaging of brain activity in addicts that are exposed to evocative
stimuli, such as an injection of a low dose of drug or stimuli (e.g., the drug
paraphernalia) that the addict associates with drug taking (Volkow and Fowler,
2000).
The Motive Circuit as a Substrate of Addiction
The circuit illustrated in Figure 13.2 consists of
interconnected nuclei that have been shown to be involved in the processing of
drug reinforcement and in initiating behaviors to obtain such reinforcement.
Neuroimaging studies have clearly identified cortical circuits that are
activated by drug-associated stimuli in addicts. This includes areas of the
prefrontal cortex, such as the anterior cingulate and the ventral orbital
cortex, as well as some allocortical regions including the amygdala (Pierce and
Kalivas, 1997; Volkow and Fowler, 2000; Grant et al., 1996; Childress et
al., 1999; Porrino and Lyons, 2000). In addition, some neuroimaging studies
have revealed involvement of the ventral striatum (including the nucleus
accumbens), espe-cially in response to a small challenge dose of drug (Porrino
and Lyons, 2000; Breiter et al.,
1997). In addition, the animal literature has identified two other brain
regions to be critical in models of primed relapse. One area is the ventral
tegmental area that, as outlined above, contains dopamine cells projecting to
the cortex and nucleus accumbens. The other region that has been associated
with stress-induced relapse is the bed nucleus of the stria terminalis, and probably
the accompanying nuclei of the extended amygdala. Finally, recent evidence has
emerged indicating a possible role for the ventral subiculum, where elec-trical
stimulation was found to induce reinstatement of drug seeking for cocaine
(Vorel et al., 2001).
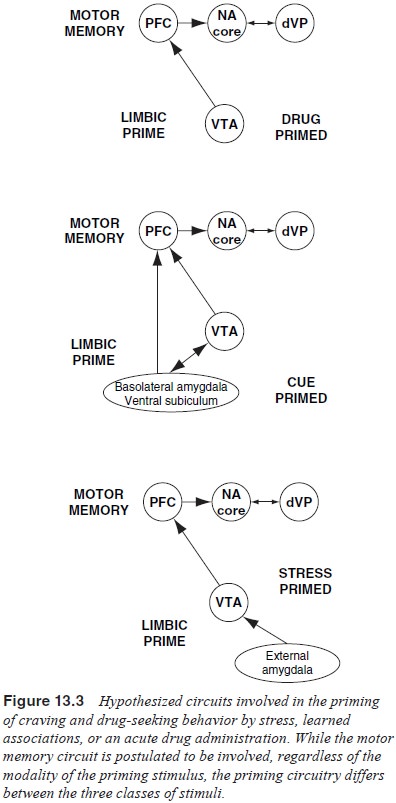
There is a growing realization that stimulus-evoked
drug-seeking behavior is comprised of two circuits, a motor memory circuit and
a limbic priming circuit (see Figure 13.2). The motor memory circuit consists
of the motor nuclei in the motive circuit including the dorsal prefrontal and
ventral orbital cortex, core of the nucleus accumbens and dorsolateral ventral
pallidum. The limbic priming circuit activates the motor memory circuit in
re-sponse to various stimuli. The stimuli activate the motor mem-ory circuit
via limbic circuitry, and the limbic nuclei involved are somewhat distinct
depending upon stimulus modality. The ventral tegmental area is integral to all
stimulus modalties, and the extended amygdala and basolateral amygdala
contribute dif-ferentially depending on whether the stimulus is a stressor or a
drug-associated cue, respectively (Figure 13.3).
Motor Memory Circuit
This portion of the circuit may be integral to all
forms of drug-taking behavior. Priming stimuli access this circuit primarily
via the prefrontal cortex and evoke behaviors organized to obtain drug reward.
Thus, the motor memory circuit functions akin to a procedural memory circuit
and, when accessed by a priming stimulus, provides a programmed sequence of
learned behaviors to obtain drug.
The regions of the prefrontal cortex most clearly
shown in neuroimaging studies to be activated by drug-associated en-vironmental
or pharmacological cues are the anterior cingulate and ventral orbital cortices
(Volkow and Fowler, 2000; Childress et
al., 1999). Both of these cortical areas make substantial gluta-matergic
projections to the nucleus accumbens (Groenewegen et al., 1996).
Although the role of the accumbens to pallidum projection in primed reinstatement is only just emerging, this projection has long been known to mediate motor activity initiated by mo-tivationally relevant stimuli (Mogenson et al., 1980, 1993; Burns et al., 1994). From this literature it is clear that both dopamine and glutamate transmission in the nucleus accumbens are nec-essary conditions for normal motor stimulation to occur (Burns et al., 1994; Robbins and Everitt, 1996; Vanderschuren and Kalivas, 2000). The data show an association between gluta-mate, but not dopamine transmission in the nucleus accumbens and drug-seeking behavior. However, in contrast to the nucleus accumbens, dopamine transmission in the dorsal prefrontal cor-tex or basolateral amygdala has been shown to be critical to the execution of cocaine- or cue-primed reinstatement, respectively (See et al., 2001; McFarland and Kalivas, 2001)
Limbic Priming Circuit
Drug-primed Reinstatement
Acute administration of a drug that was previously
self-administered is known to elicit craving and drug-seeking behaviors in
experimental animals and human addicts (O’Brien 2001; Markou et al., 1993). The role of dopamine at
inducing priming is well established since both systemic and intra-cortical
administration of dopamine blockers have been shown to inhibit drug-primed
reinstatement. However, activation of dopamine neurons is apparently not a
prerequisite, although having an intact dopamine system is permissive. Figure
13.3 illustrates the circuit critical for cocaine-primed reinstatement. It is
proposed that this is a minimal circuit and that other drugs of abuse may
involve additional brain nuclei that activate dopamine cells. For example,
disinhibition of GABAergic input to dopamine cells in the ventral tegmental
area by a microinjection of morphine is known to elicit reinstatement in
animals trained to self-administer heroin (Stewart, 1984).
Cue-primed Reinstatement
When animals or humans experience drug effects in
the presence of an environmental stimulus (cue), a learned association develops
such that presentation of that cue will elicit craving and behavior organized
to obtain drug reward. Cue-primed reinstatement of drug-seeking behavior is clearly
dependent upon the functional integrity of the basolateral amygdala (Meil and
See, 1997; Grimm and See, 2000). This region of the amygdala has also been
shown to be critical for many forms of stimulus-reinforcer associations
(Everitt et al., 1999). Moreover, it
was recently shown that in a manner analogous to the role of the prefrontal
cortex in drug-primed reinstatement, blockade of D1 dopamine
receptors in the basolateral amygdala prevents cue-primed reinstatement (See et al., 2001). This effect implies that
presentation of the cue activates the projection from the ventral tegmental
area to the basolateral amygdala, and is consistent with a well-developed
electrophysiological literature showing that dopamine neurons in the ventral
tegmental area increase burst firing following presentation of a cue that
predicts a reward (Schultz, 1998).
Stress-primed Reinstatement
Addicts often report that environmental stress can
precipitate craving and drug-taking behavior (O’Brien, 2001; Lyvers, 2000). The
regions of the brain most clearly associated with stress-primed reinstatement
are associated with extended amygdala (Shaham et al., 2000; Heimer et al.,
1993).
Integration of Findings
Present knowledge suggests the possibility of a
final common pathway for addiction, and possibly similar brain circuits between
drugs and stimuli that provoke craving and relapse. The extant data support a
common role of the motor memory circuit shown in Figure 13.3 that consists of
the series projection from the prefrontal cortex to nucleus accumbens to
ventral pallidum. Moreover, there is abundant evidence for enduring
neuroadaptations in gene expression and neuronal function in the nucleus
accumbens and prefrontal cortex following a bout of drug taking (Nestler, 2001;
Kalivas, 2002). While the finding from multiple lines of research are promising
in pointing towards a common site of intervention in addiction, it is important
to note that such a generalization based primarily on work with
psychostimulants is premature and requires substantially more research using
other classes of drugs to validate. Similarly, the proposal for a final common
motor memory pathway mediating craving and relapse induced by different
modalities of stimuli is based on only a modest number of neuroimaging studies
in addicts and experimental models of relapse. Nonetheless, sufficient
supportive data has accrued to at least speculate on the prepotent involvement
of the motor memory pathway in addiction, especially the glutamatergic
projection from regions of the prefrontal cortex including the anterior
cingulate and ventral orbital cortex to the core of the nucleus accumbens.
Likewise, dopamine projections to prefrontal cortex and allocortical areas such
as the basolateral amygdala are also critical.
These emerging hints and hypotheses pose directions
for novel pharmacological therapeutic strategies for ameliorat-ing craving and
relapse associated with addiction. Notably, pharmacological regulation of
glutamate transmission in the cor-tical projection to the nucleus accumbens
would seem to be a po-tential target. Given that enhanced release of glutamate
appears to be associated with cue-, drug-, and perhaps, stress-primed re-lapse,
diminishing that release would be one possible mechanism for pharmacotherapeutic
intervention.
Related Topics