Chapter: Essentials of Psychiatry: Psychiatric Pathophysiology: Addiction
The Development of Addiction
The Development of Addiction
The acute administration of all addictive drugs,
with the pos-sible exception of the benzodiazepines, stimulates dopamine
transmission in the projection from the ventral mesencephalon to the nucleus
accumbens. This projection is generally re-ferred to as the mesolimbic dopamine
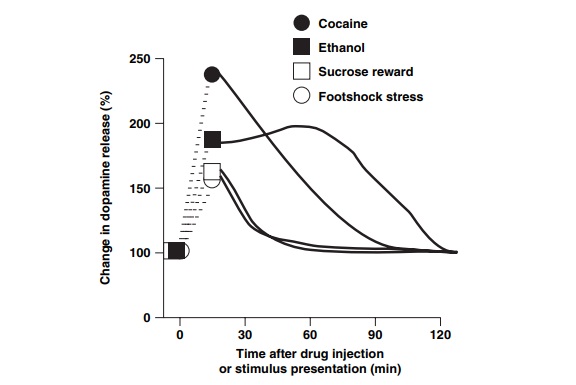
system; Figure 13.1
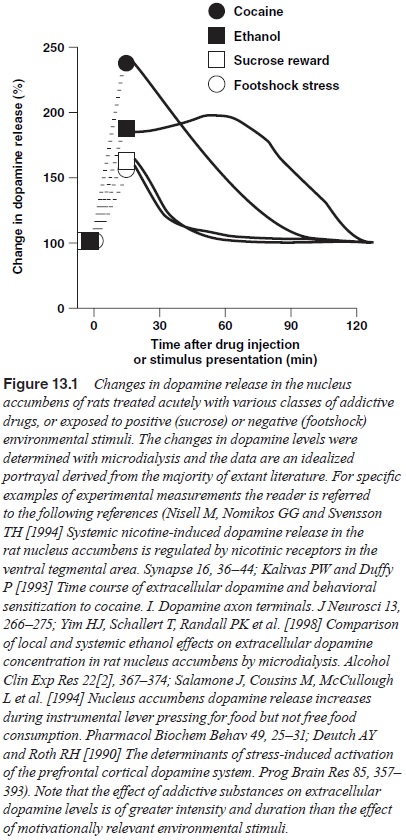
illustrates the capacity of two drugs of abuse –
cocaine and ethanol – to increase dopamine transmission in the nucleus
accumbens. The pharmacological site of action by which different classes of
drugs of abuse activate dopamine trans-mission varies and includes three
general cellular mecha-nisms that encompass all drugs of abuse: 1) Receptors
for the drug are on dopamine cell bodies and dendrites, and through these
receptors drug administration directly stimu-lates dopamine neurons. Nicotine
and marijuana are exam-ples of drugs working in part through this mechanism
(Cheer et al., 2000; Nisell et al., 1994). 2) Receptors are located
prima-rily on GABAergic inhibitory afferents to the dopamine cells, and drug
binding to these receptors reduces GABA release, thereby disinhibiting dopamine
neuronal activity. Opioids and ethanol produce reward in part by this mechanism
(Bunney et al., 2001; Cameron et al., 1997). 3) Finally, drugs can
bind to presynaptic receptors to
increase the presynaptic release of dopamine without directly altering the
activity of dopamine neurons. The primary mechanism in this category is
exem-plified by amphetamine-like psychostimulants which bind to the dopamine
transporter and increase dopamine release by blocking reuptake and/or promoting
the release of dopamine by reverse transport (Seiden et al., 1993).
Figure 13.1 also illustrates that mesolimbic
dopamine is released by environmental stimuli that are motivationally rel-evant
to the organism, regardless of the valence of the stimulus. For example, either
positive motivational stimuli such as sex and food, or negative stressful
stimuli will increase dopamine release. However, as indicated in Figure 13.1,
regardless of the intensity of the natural stimulus, the extent of dopamine
release is far less than that produced by drugs. This is especially true for
the duration of dopamine release induced by a typical dose of ad-dictive drugs
that will endure for many minutes to hours, while a natural stimulus is thought
to elevate dopamine for a period of only a few minutes, even if the stimulus
itself is present for a greater length of time.
Unlike physiological stimuli, the increase in
dopamine transmission does not diminish following repeated drug administration
(although this varies with drug class, and some tolerance can be demonstrated
to all drugs during a binge of drug taking). Thus, repeated drug use is
associated with repeated increase in dopamine, thereby providing a repeated
stimulus for cellular adaptation. Due to both the relative lack of tolerance to
drug-induced dopamine release as well as to the fact that the quantity of
release is well in excess of what is seen physiologically with naturally
rewarding stimuli (see Figure 13.1), it is thought that dopamine-dependent
neuroplastic changes induced by repeated drug use are beyond the physiological
range of normal cellular adaptation. This pathological event precipitates a
sequence of cellular changes that ultimately produce neuroadaptations that are
widespread in cortical and limbic circuitry, and these adaptations constitute
the underlying pathophysiology of addiction. Although the addiction-associated
neuroadaptations are impacted by drug class, dose and withdrawal period, it is
thought that excessive, nonphysiological release of dopamine is a critical
initiator of the pathology.
Identifying the specific changes in gene expression and cellular function that are associated with the development of ad-diction is an area of active research, and the interested reader is referred to recent reviews of this literature (Nestler, 2001; Kalivas, 2002).
Related Topics