Chapter: Medical Physiology: Protein Metabolism
Basic Properties of Protein Metabolism: Amino Acids
Basic Properties
Amino Acids
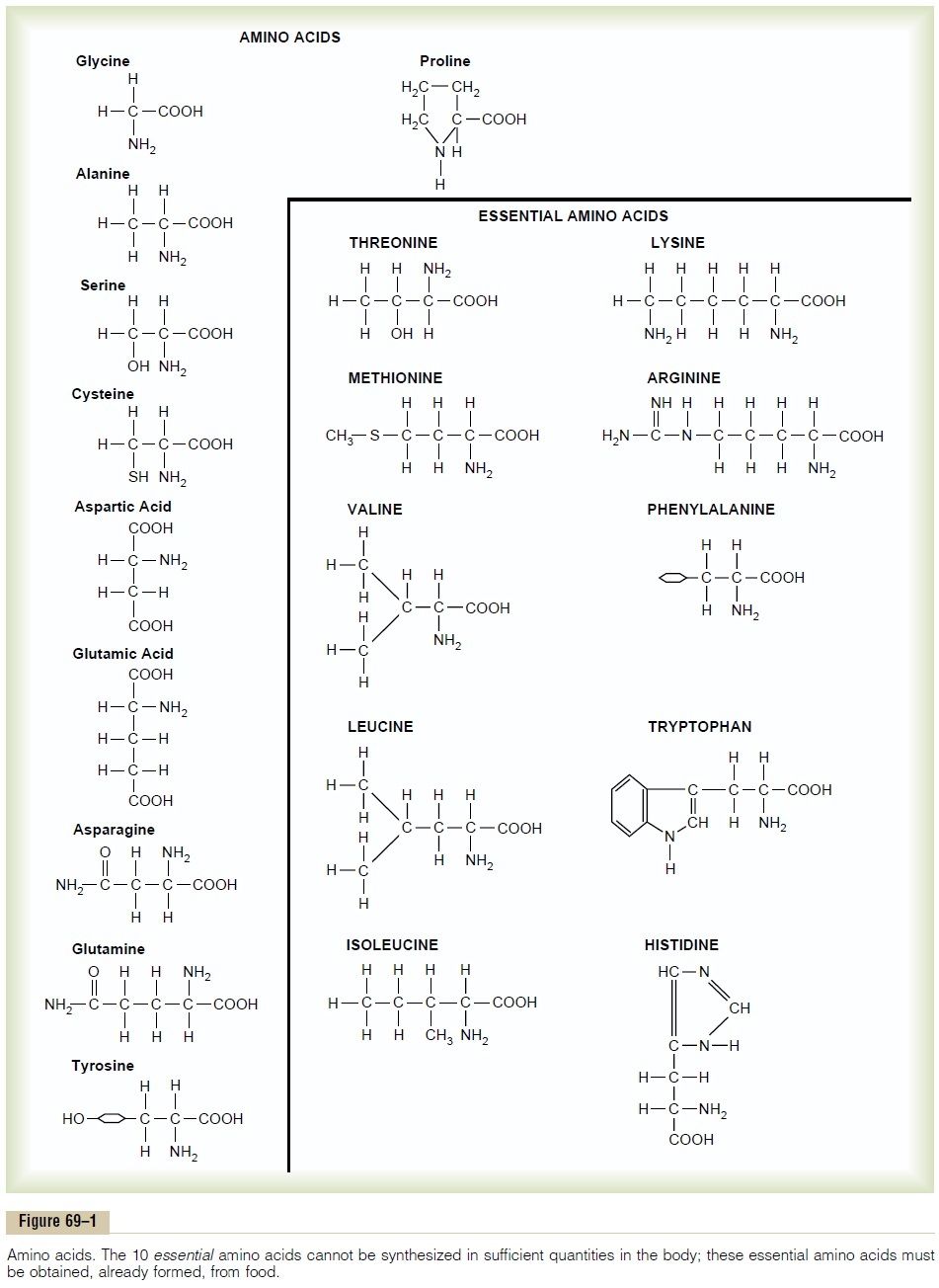
The principal constituents of proteins are amino acids, 20 of which are present in the body proteins in significant quantities. Figure 69–1 shows the chemical formulas of these 20 amino acids, demonstrating that they all have two features in common: each amino acid has an acidic group (—COOH) and a nitrogen atom attached to the molecule, usually represented by the amino group (—NH2).
Peptide Linkages and Peptide Chains. In proteins, the amino acids are aggregated intolong chains by means of peptide linkages. The chemical nature of this linkage is demonstrated by the following reaction:
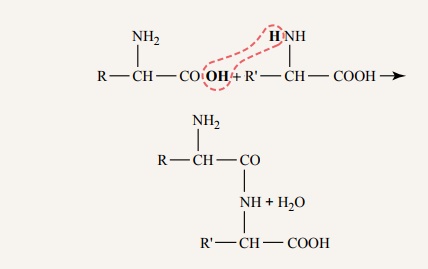
Note in this reaction that the nitrogen of the amino radical of one amino acid bonds with the carbon of the carboxyl radical of the other amino acid. A hydrogen ion is released from the amino radical, and a hydroxyl ion is released from the car-boxyl radical; these two combine to form a molecule of water. After the peptide linkage has been formed, an amino radical and a carboxyl radical are still at oppo-site ends of the new, longer molecule. Each of these radicals is capable of combin-ing with additional amino acids to form a peptide chain. Some complicated protein molecules have many thousand amino acids combined by peptide linkages, and even the smallest protein molecule usually has more than 20 amino acids combined by peptide linkages. The average is about 400 amino acids.
Other Linkages in Protein Molecules. Some protein molecules are composed of severalpeptide chains rather than a single chain, and these chains are bound to one another by other linkages, often by hydrogen bonding between the CO and NH radicals of the peptides, as follows:
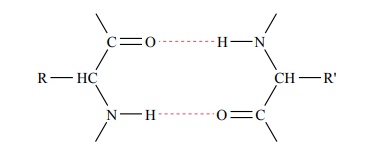
Many peptide chains are coiled or folded, and the suc-cessive coils or folds are held in a tight spiral or in other shapes by similar hydrogen bonding and other forces.
Related Topics