Chapter: Pharmaceutical Drug Analysis: Liquid-Liquid Extraction
Assay Methods Based on Liquid-Liquid Extraction
ASSAY METHODS BASED ON LIQUID-LIQUID EXTRACTION
A number of specific elements may be determined
quantitatively based on liquid-liquid extraction method or ‘solvent-extraction’ technique, namely :
(a)
Determination of copper (I) as the neo-cuproin complex,
(b)
Determination of Iron (III) as the 8-hydroxyquinoline complex or Iron (III)
oxinate,
(c)
Determination of lead (I) by the dithizone method,
(d)
Determination of molybdenum (VI) by the thiocyanate method, (e) Determination of Ni (II) :
(i) as
dimethylglyoxime complex, and
(ii) by
synergistic extraction.
All these assay methods shall be discussed in the
following sections :
1. DETERMINATION OF COPPER (I) AS THE NEO-CUPROIN COMPLEX
Theory
‘Neo-cuproin’ (i.e., 2, 9-dimethyl-1 :
10-phenathroline) under specific experimental parameters almost behaves as a critical reagent for
copper (I). The resulting complex is freely soluble in chloroform and absorbs
at 457 nm.
Materials Required : hydroxyammonium chloride
solution (10% w/v) : 25 ml ; sodium citrate solution (30% w/v) : 50 ml ; ammonia solution ; ‘neo-cuproin’ solution (0.1% w/v in absolute ethanol) : 50 ml ;
chloroform ;
Procedure : The following steps may be
adopted :
·
Transfer 10.0 ml of the sample solution (containing upto
200 mcg of copper) in a separatory funnel, add 5 ml of hydroxyammonium chloride
solution to affect the reduction of Cu (II) to Cu (I),
·
To the resulting solution add 10 ml of solution citrate
solution to enable complexation of any other metals that may be present,
·
Add ammonia solution gradually until the pH is about 4.0
(use Congo Red) followed by 10 ml ‘neo-cuproin’
solution,
·
Shake for about 30 seconds with 10 ml of chloroform and
allow the layers to separate,
·
Repeat the extraction with a further 5 ml of chloroform,
and
·
Finally, measure the absorbance at 457 nm against a blank
on the reagents that have been used identically to the sample.
2. DETERMINATION OF IRON (III) AS THE 8-HYDROXY QUINOLATE COMPLEX [IRON (III) OXINATE]
Theory : Iron (III) upto an extent of
50-200 mcg can be extracted effectively from an aqueous solution with a 1% solution of
8-hydroxyquinoline (symbolized as HQ) in chloroform by carrying out a double
extraction when the pH of the resulting aqueous solution ranges between 2 and
10. Evidently, between pH 2.0 to 2.5 metals like Ni, Co, Ce (III) and Al do not
interfere at all. However, iron (III) oxinate is dark-coloured in chloroform
and absorbs at 470 nm.
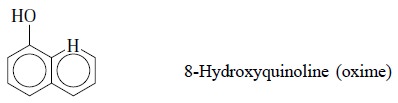
The reaction may be expressed as follows :
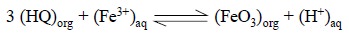
Materials Required : Hydrated ammonium iron (III)
sulphate : 0.0266 g ; oxine solution (‘AnalaR’-Grade, 1% w/v in chloroform) :
50 ml ; chloroform ; 100 ml ;
Procedure : The following steps may be
followed :
·
Weigh accurately 0.0226 g of hydrated ammonium iron (III)
sulphate and dissolve it in 1 litre of DW in a volumetric flask ; 50 ml of this
solution contains 100 mcg of iron,
·
Place 50 ml of the solution (≡
100 mcg of Fe) in a 100-ml separatory funnel, and add to it 10 ml of 1% oxine
solution, and shake for 1 minute,
·
Separate the chloroform layer,
·
Transfer a portion of the chloroform layer to a 1 cm
absorption cell and determine the absorbance at 470 nm in a
UV-spectrophotometer, employing the solvent (chloroform) as a blank or
reference, and
·
Repeat the extraction with a further 10 ml quantity of 1%
oxine solution, and measure the absorb-ance again so as to confirm whether all
the iron was extracted or not. Usually three extractions suffice the complete
extraction of Fe (III).
Note : From a glimpse of
typical analytical results it may be seen that absorbance after first
extraction 0.0592 ; after second extraction 0.0050 ; after third extraction
0.0010 ;
3. DETERMINATION OF LEAD (I) BY THE DITHIZONE METHOD
Theory : In solution, dithizone
(diphenylthiocarbazone) exhibits tautomerism as shown below :
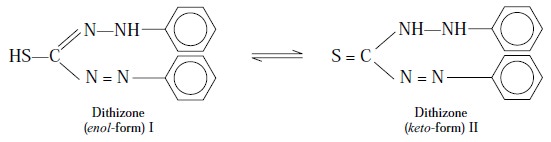
The enol-form of Dithizone (I) behaves
as monoprotic acid having a dissociation constant pKa = 4.7 upto a pH
range of about 12 : obviously, the acid proton is inherited due to the thiol
moiety in (I). In reality, two kinds of ‘metal
dithizonates’ are invariably formed, namely :
(a)
‘Primary’ metal dithizonates : These are produced as per the
following reaction :
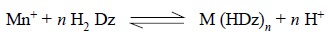
They are of greater analytical value because of their
high stability and greater solubility in organic solvents.
(b) ‘Secondary’ metal dithizonates : These
are specifically formed by some metals, such as : Cu, Ag, Au, Hg, Bi and Pd.
The second complex are produced under the following two conditions, namely :
(i) deficiency
of the reagent, and
(ii) higher pH
range,
and may be expressed as follows :
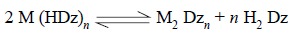
It is, however, pertinent to mention here that dithizone*
is an extremely sensitive reagent and, there-fore, helps in the determination
of lead either from a neutral or faintly alkaline medium to the extent of a few
micrograms.
Materials Required : Pure lead nitrate : 0.0079 g ;
ammonia-cyanide-sulphite mixture (dilute 35 ml of conc. ammonia solution having sp. gr. 0.88 and 3 ml of 10% w/v
solution potassium cyanide (Caution :
deadly poisonous, use protective gloves
while handling) to 100 ml, and then dissolving 0.15 g of sodium sulphite in this solution) : 75 ml ;
dithizone (pure) solution (0.005% w/v in chloroform)** : 7.5 ml ; chloroform :
17.5 ml ;
Procedure : Dissolve 0.0079 g of pure lead
nitrate in 1 litre of DW in a volumetric flask. To 10 ml of this solution (equivalent to about 50 mcg of Pb) contained in a
250-ml separatory funnel, add 775 ml of ammonia-cyanide-mixture, and adjust the
pH of the resulting solution to pH 9.5 by the careful addition of HCl. Now, add
7.5 ml of dithizone solution and 17.5 ml of chloroform rapidly. Shake the
contents of the separatory funnel thoroughly for 1 minute, and allow the phases
to separate. Determine the absorbance at 510 nm vis-a-vis a blank solution in a 1.0 cm absorption cell. However, a
further extraction of the same solution yields zero absorption thereby
indicating that complete extraction of lead has taken place.
4. DETERMINATION OF MOLYBDENUM (VI) BY THE THIOCYANATE METHOD
Theory : Molybdenum (VI) is mostly
converted to molybdenum (V) when an acidic solution of the former is treated with tin (II) chloride preferably in the
presence a little Fe2+ ion. The resulting molybdenum (V) form a red
complex with thiocyanate ion as follows :
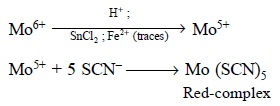
Consequently, the red-complex is extracted with either
solvents possessing donor oxygen atoms, such as : 3-methyl butanol. However, Mo
(VI) may also be extracted with diethyl ether-an oxygenated solvent, because it
yields the maximum percentage extractive with 7.0 M NH4 SCN as could
be seen from the follow-ing Table 27.2.
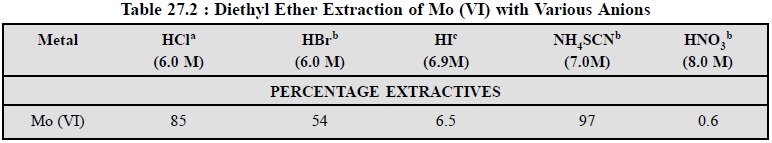
a-3 : 2 volume ratio of organic
to aqueous phase
b-1 : 1 volume ratio of organic
to aqueous phase
c-4 : 1 volume ratio of organic
to aqueous phase
The molybdenum complex exhibits maximum absorption at 465
nm.
Materials Required :
(i) Standard Molybdenum Solution : Dissolve
0.184 g of ammonium molybdate (NH4)6 [Mo7O24]
4H2O in 1 litre of distilled water in a volumetric flask : this
yields a 0.01% solution which can be diluted to 0.001% with 0.1 M HCl, thereby
giving a Mo solution containing 100 mcg ml–1,
(ii) Ammonium Iron (II) Sulphate Solution :
Dissolve 10 g of the salt in 100 ml of very dilute sulphuric acid,
(iii) Tin (II) Chloride Solution : Dissolve
10 g of Tin (II) chloride dihydrate in 100 ml of 1 M HCl, and
(iv) Potassium Thiocyanate Solution :
Prepare a 10% w/v aqueous solution from the pure salt (‘AnalaR’-Grade).
Procedure : The various steps involved are
as follows :
1)
First of all construct a calibration curve by
transferring accurately 1.0, 2.0, 3.0, 4.0 and 5.0 ml of the 0.001% Mo solution
(i.e., containing 10, 20, 30, 40, and
50 mcg Mo respectively) in individual 50-ml separatory funnels and diluting
each of them with an equal volume of water.
2)
Add to each funnel 2 ml of conc. HCl, 1 ml of ammonia
iron (II) sulphate solution, and 3 ml of the potassium thiocyanate solution,
3)
Shake gently and then induce 3ml of the tin (II) chloride
solution,
4)
Add water to bring the total volume in each separatory
funnel to 25 ml and mix thoroughly,
5)
Introduce exactly 10 ml of redistilled 3-methyl butanol
into each funnel and shake them separately for 30 seconds,
6)
Allow the two phases to separate completely and carefully
drain out the lower aqueous layer,
7)
Remove the glass-stopper and pour the alcoholic extract
through a small plug of purified glass wool in a small funnel and transfer the
organic extract to a 1 cm absorption cell,
8)
Measure the absorbance at 465 nm in a UV
spectrophotometer against a 3-methyl butanol blank,
9)
Plot the graph by taking absorbance against concentration
of Mo in Mcg, thereby obtaining a straight line spreading over a range 0-50 mcg
of Mo (obeying Beer’s Law), and
10)
Finally, determine the concentration of Mo in unknown
samples provided and containing less than 50 mcg Mo per 10 ml ; make use of the
calibration curve, and subject the unknown samples to the same treatment as the
standard solutions.
5. DETERMINATIONS OF NICKEL (II)
A. As Dimethylglyoxime Complex
Theory : In ammoniacal solution, Ni
(II) forms an insoluble red coordination compound with dimethylglyoxime (C4H8O2N3).
Nickel dimethylglyoximate is only sparingly soluble in chloroform (35-50 mcg Ni
ml–1). It is, however, necessary to know the approximate amount of
Ni present in the sample, so as to avoid adding a large excess of
dimethylglyoxime, which is not very soluble in water and may precipitate easily
along with the nickel-complex. The optimum pH range at which the extraction of
this complex should be carried out ranges between 7-12 in the presence of
citrate. It has been observed that the nickel-complex is quite bulky in nature
when first precipitated and hence, shows a tendency to move up along the walls
of the container. Therefore, care should be taken that the sample must not
contain more than 50 mg of Ni. Lastly, the nickel complex absorbs at 366 nm and
also at 465-470 nm.
The formation of nickel dimethylglyoximate complex may be
expressed as follows :
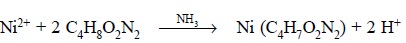
Materials Required : Ammonium nickel sulphate
(pure) : 0.135 g ; citric acid : 5.0 g ; dilute ammo-nia solution ;
dimethylglyoxime solution (dissolve 0.50 g of dimethyl-glyoxime in 250 ml of
ammonia solu-tion and diluting to 500 ml with water) : 20 ml ; chloroform : 50
ml ; Procedure
·
Weigh accurately 0.135 g of pure ammonium nickel sulphate
(NiSO4, (NH4)2 SO4, 6H2O)
and dissolve in 1 litre of distilled water in a volumetric flask,
·
Transfer 10 ml of the resulting solution (Ni ~– 100 mcg)
into a breaker containing 90 ml of water,
·
Add to it 5 g of citric acid, and then dilute ammonia
solution carefully until the pH is 7.5,
·
Cool and transfer to a separatory funnel, add 20 ml of
dimethylglyoxime solution and, after stand-ing for a minute 12 ml of
chloroform,
·
Shake the contents of the funnel for 1 minute, permit the
two phases to separate out completely,
·
Collect the lower red chloroform layer and determine the
absorbance at 366 nm in a 1 cm absorp-tion cell against a blank, and
·
Extract once again with a 12 ml portion of chloroform and
measure its absorbance at 366 nm ; usually very negligible Ni (II) may be
found.
B. Synergistic Extraction
Theory : Dithizone and 1,
10-phenanthroline help in the synergistic extraction of Ni (II) both quantitatively and rapidly over a wide range of pH
between 5.5 to 11.0 to give rise to a dark coloured mixed-ligand complex that
absorbs at 520 nm. The resulting complex bears the following vital
characteristic features, namely :
(i) It is
fairly stable to allow the complete removal of excess dithizone by
back-titration with 0.1 M NaOH, so as to make a ‘monocolour’ method feasible,
(ii) The molar
absorptivity of the complex stands at 4.91 × 10 4 mol–1 L
cm–1, and
(iii) The
synergistic method is predominantly much more sensitive as compared to any
other method for the determination of Ni (II).
Materials Required : Ammonium nickel sulphate*
(pure) : 0.0135 g ; phthalate or acetate (ethanoate)
buffer (pH 6.0) : 5 ml ; dilute ammonia solution ;
chloroform : 15 ml ; sodium hydroxide (0.1 M) : 10.0 ml ;
Procedure
·
To 5 ml of a solution containing from 1 to 10 mcg of
Nickel (II) 5 ml of a phthalate or acetate buffer,
·
In case, the sample is acidic, adjust the pH to 6.0 with
dilute ammonia solution carefully,
·
To the resulting solution add 15 ml of chloroform
solution of dithizone and 1, 10-phenanthroline,
·
Moderately shake the two phases for 5 minutes in a separatory
funnel, allow them to separate distinctly into aqueous and chloroform (lower)
layers,
·
Excess dithizone may be removed from the chloroform layer
by back-extraction with 10 ml of 0.1 M NaOH, (a through shaking for 60 seconds
will suffice this extraction),
·
Once again separate the chloroform layer (lower) and
measure its absorbance in a 1 cm absorption cell at 520 nm Vs an identically treated blank, and
·
Finally, draw a calibration curve using standard Ni (II)
solution containing 2, 4, 6, 8, and 10 mcg in 10 ml (obeying Beer’s Law).
Caution : All glassware must be rinsed
with dilute acid and then thoroughly with distilled water.
Related Topics