Chapter: Pharmaceutical Drug Analysis: Aqueous Titrations
Aqueous Titrations
AQUEOUS TITRATIONS
INTRODUCTION
Arrhenius’ definition of an acid is—‘a substance which
yields hydrogen ion (H+) in an aqueous medium’; and that of a base is—‘a substance which yields hydroxy ions (OH–) in an aqueous
medium’.
However, these definitions have two serious short-comings, they are :
(a) they lack
explanation of the behaviour of acids and bases in non-aqueous media, and
(b) acidity is associated with hydrogen ion—a relatively simple
particle ; whereas, basicity is
associated with hydroxyl ion—a
relatively complex entity.
1. LOWRY AND BRONSTED’S THEORY OF ACIDS AND BASES
Just after the First World War in 1923, Bronsted and
Bjerrum in Denmark and Lowry in Great Britain jointly put forward a more
acceptable and satisfactory theory of acids and bases which is devoid of
objec-tions earlier raised in Arrhenius’ definition.
According to Lowry and Bronsted’s theory—‘an acid is a substance capable of yielding
a proton (hydrogen ion), while a
base is a substance capable of accepting a proton’. Thus, a complementary relationship exists between an acid
and a base that may be expressed in a generalized fashion as below :
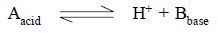
1.1. Conjugate Acid-Base Pair
The pair of substances which by virtue of their mutual
ability either gain or lose a proton is called a conjugate acid-base pair. A few typical examples of such pairs are
:
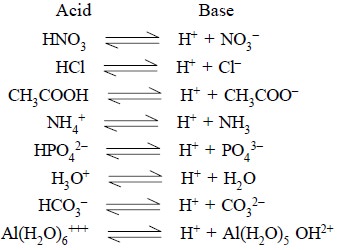
It is quite evident from the above examples that not only
molecules but also anions and cations can act as acids and bases.
In an acid-base titration, the acid will not release a
proton unless the base capable of accepting it is simultaneously present ; in
other words, in a situation where actual acid-base behaviour exists then an
interaction should involve two sets of conjugate acid-base pairs, represented
as :
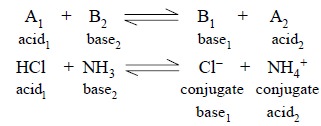
Some other examples include :
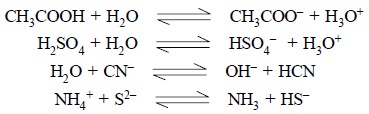
In short, the species which essentially differ from each
other by one proton only, are known as conjugate
base and acid respectively.
Sometimes, such a reaction is termed as
protolytic reaction or protolysis, where A1 and B1
make the first conjugate acid-base pair and A2 and B2 the
other pair.
1.2. Merits of Lowry-Bronsted Theory
It has two points of merit, which are :
(a)
hydrochloric acid on being dissolved in water undergoes a
protolytic reaction, thus :
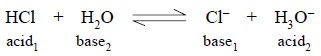
It may be observed that H3O+, known
as hydronium or oxonium ion is invariably formed when an acid is dissolved in
water.
Likewise, ammonia on being dissolved in water is also
subjected to protolysis, thus :
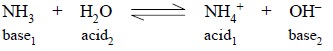
(b)
all proton-transfer reactions may be handled, thus :
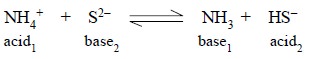
1.3. Demerits of Lowry-Bronsted Theory
It does not hold good for nonprotonic solvents, for instance : BF3, POCl3
and SO2.
2. LEWIS’S THEORY
Lewis (1923) put forward another definition of acids and
bases solely dependent on giving or taking of an electron pair. According to
Lewis—‘an acid is an electron pair
acceptor, whereas a base is an electron
pair donor’. Therefore, it is obvious that whenever any neutralization
occurs the formation of an altogether
new coordinate covalent bond between the electron pair donor and acceptor atoms
take place.
Thus, Lewis’s definition is a much broader definition
that includes coordination compound formation as acid-base reactions, besides
Arrhenius and Lowry-Bronsted acids and bases. Examples :
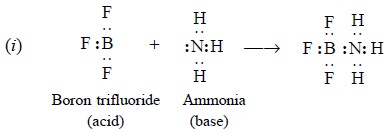
The reaction of borontrifluoride (acid) with ammonia
(base) results into a stable octet configuration between mutual sharing of a
pair of electrons of latter (donor) and former (acceptor).
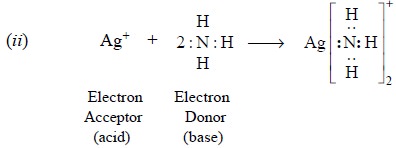
The reaction of ammonia (base) with Ag+ (acid)
results into a stable configuration due to the mutual sharing of a pair of
electrons of latter (donor) and former (acceptor).
3. USANOVICH THEORY
Usanovich (1934) modified the Lewis concept of acid and
base by removing the restriction of either donation or acceptance of the
electron pair in a more generalized fashion. According to him :
Acid : It is a chemical species that reacts with a base thereby
giving up cations or accepting anions or electrons.
Base : It is a chemical species that reacts with an acid thereby
giving up anions or electrons or combines with cations.
Unlike Arrhenius, Lowry-Bronsted and Lewis acids and
bases, the Usanovich’s concept in a much broader sense includes all the oxidizing
agents as acids and the reducing agents as bases, e.g.,
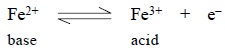
In the Iron (II)—Iron (III) system, the ferric ion (III)
acts as an oxidizing agent and is an acid ; while the ferrous ion (II) acts as
a reducing agent and is a base.
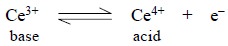
Similarly, in the Cerous (III)—Ceric (IV) system, the
ceric ion (IV) behaves as an oxidizing agent and acts as an acid ; while the
cerous ion (III) behaves as a reducing agents and acts as a base.
4. LUX-FLOOD CONCEPT
The concept of acid-base reactions with respect to the
oxide ion was first introduced by Lux (1929) and supported by Flood (1947).
According to the Lux-Flood concept—‘an
acid is the oxide-ion acceptor while
a base is the oxide donor’. Examples :
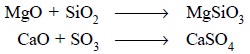
In the above reactions both MgO and CaO are the oxide ion
donor and hence act as bases, whereas SiO2 and SO3 are
the oxide-ion acceptor and hence act as acids. Ultimately, the Lux-Flood acid
and base react to form magnesium silicate (MgSiO3) and calcium sulphate
(CaSO4) salts respectively.
Related Topics