Chapter: Pharmaceutical Drug Analysis: Aqueous Titrations
Theory of Alkalimetry
THEORY OF ALKALIMETRY
Acidic substances are usually determined quantitatively
by methods similar to those used for the quantitative determinations of bases.
However, two methods are generally
adopted for the assay of acidic substances, namely :
(a) Direct Titration Methods : It is
accomplished by directly titrating an exact quantity of the acid, acid salt or
other acidic substance with standard alkali solutions.
(b) Residual Titration Methods : It is
carried out by the addition of an excess of the standard alkali solution and
subsequently determining the amount in excess by residual titration with standard
acid solution.
As a general principle, the following guidelines may be
observed carefully, namely :
(i) the
normality of the solution obtained by dissolving the acidic substance must be
approximately the same as that of the titrant,
(ii) the liquid
acidic substance to be titrated must be brought to room temperature (25°C)
before titration, because many indicators offer different values at different
temperatures, and
(iii) the
quantity of acid to be taken should be calculated in such a manner that approximately
30 to 40 ml of the previously standardized base shall be utilized for the
assay.
Inorganic Acids—for these either methyl red
or phenolphthalein may be employed as indicators and the alkali must be standardized with the particular indicator used.
Organic acids—for these phenolphthalein is
invariably used, but bromothymol blue, thymol blue and thymolphthalein are also employed as per specific
requirements.
Besides, the aforesaid visual methods of assay i.e., observing the change in colour of
indicators used, alternative instrumental methods such as : potentiometric,
amperometric, polarographic, conducto-metric methods are also employed in
determining the end-point.
1. DIRECT TITRATION METHOD (DTM)
1.1. Tartaric Acid
Materials Required : 2 g of Tartaric acid ; 1 N
sodium hydroxide.
Procedure : Place 2 g of previously dried
and accurately weighed sample of tartaric acid in a conical flask. Dissolve it in 40 ml of DW, add a few drops of
phenolphthalein indicator and titrate with standardized 1 N sodium hydroxide.
Each millilitre of 1 N sodium hydroxide is equivalent to 75.04 mg of C4H6O6.
Equation :
H2C4H4O6 +
2NaOH → Na2C4H4O6 +
2H2O
(150.09)
From the above equation it is evident that two moles of
sodium hydroxide is needed to neutralize one mole of tartaric acid, therefore,
the equivalent weight of tartaric acid is 75.04 g. Hence, each millilitre of 1
N sodium hydroxide is equivalent to 0.07504 g (i.e., 1 meq) of tartaric acid.
Thus, the percentage of tartaric acid present in the
sample is given by :
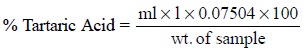
1.2. Busulphan
Materials Required : 0.25 g of Busulphan ; 0.1 N
sodium hydroxide.
Procedure : Weigh accurately about 0.25 g
of busulphan, add 25 ml of DW and boil gently under a reflux condenser for 30 minutes. Wash the condenser with a small
quantity of DW, cool and titrate with 0.1 N sodium hydroxide using
phenolphthalein solution as indicator. Each millilitre of 0.1 N sodium
hydrox-ide is equivalent to 0.01232 g of C6H14O6S2.
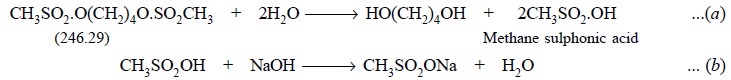
Busulphan is first hydrolyzed by refluxing it with water
and two moles methanesulphonic acid (from one mole of Busulphan) thus
generated, titrated with 0.1 N sodium hydroxide employing phenolphthalein as
indicator. Hence, the equivalent weight of busulphan is 123.145 g. Therefore,
each millilitre of 0.1 N sodium hydroxide is equivalent to 0.01232 g of
busulphan.
Thus, the percentage of busulphan present in the sample
may be calculated as under :
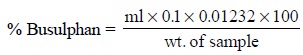
Caution : Busulphan is
extremely poisonous. Great care should be taken to avoid inhaling the particles
of busulphan or exposing the skin to it.
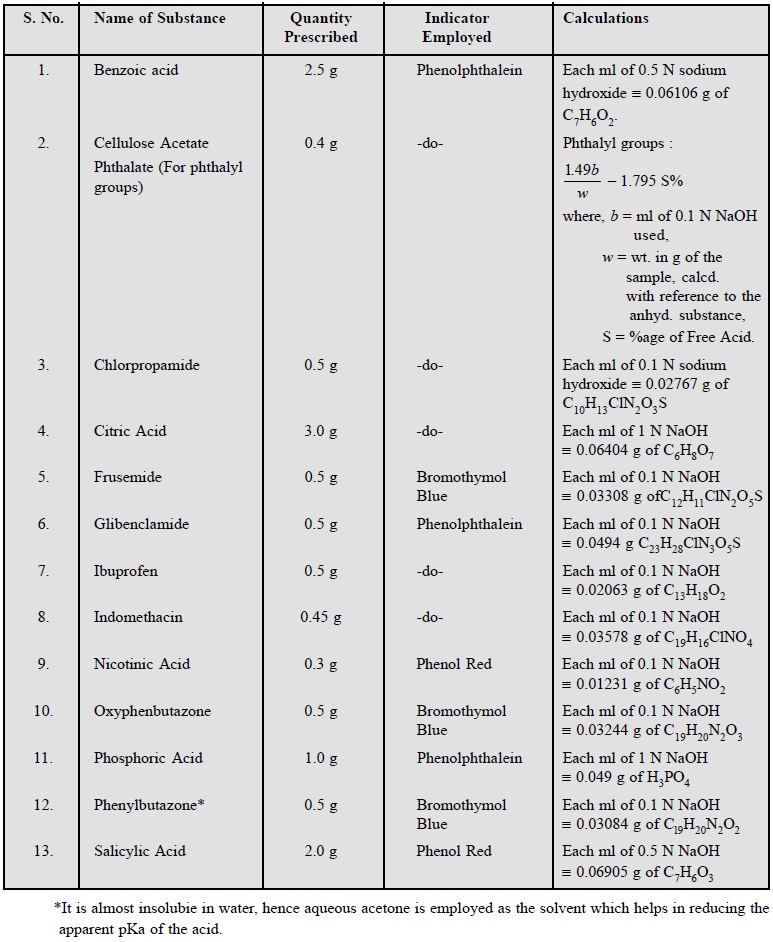
1.3. Cognate Assays
Benzoic acid ; cellulose acetate phthalate ;
chlorpropamide ; ibuprofen ; indomethacin ; nicotinic acid ; oxyphenbutazone ;
phosphoric acid ; phenylbutazone and salicylic acid.
2. RESIDUAL TITRATION METHODS (RTM)
This method is mostly applicable to official compounds belonging to the class of esters, acid
anhydrides, aldehydes and acid chlorides. In practice this method applies to
such substances that normally react too slowly with the titrant because of
their poor solubility which may be accomplished either by a heating process or
by a precipitation method so as to convert the substance capable for reaction
with the standard base.
2.1. Aspirin Tablets
Materials Required : 20 Aspirin Tablets ; 0.5 N
sodium hydroxide ; 0.5 N HCl.
Procedure : Weigh and powder 20 tablets.
Accurately weigh a quantity of the powder equivalent to about 0.5 g of aspirin, add 30.0 ml of 0.5 N sodium hydroxide
boil gently for 10 minutes and titrate with 0.5 N hydrochloric acid using
phenol red solution as an indicator. Repeat the operation without the substance
being examined, the difference between the titrations represents the amount of
0.5 N sodium hydroxide required by the aspirin. Each ml of 0.5 N sodium
hydroxide is equivalent to 0.04504 g of C9H8O4.
Equations : The aspirin is titrated with
sodium hydroxide so as to neutralize any free acid formed by hydrolysis of the acetylsalicylic acid
as shown by the following equation :
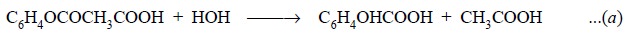
The carbonyl group present in acetylsalicylic acid is
subsequently neutralized with NaOH to yield :
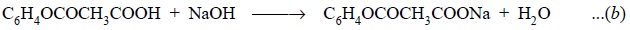
Further reaction of aspirin with excess of standard NaOH
added followed by heating results in the saponification of the sodium
acetylsalicylate as shown below :
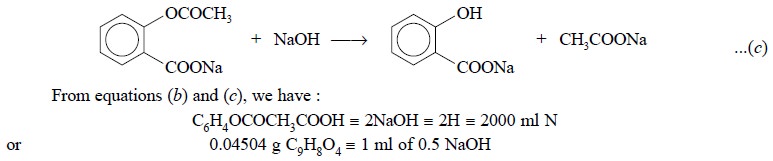
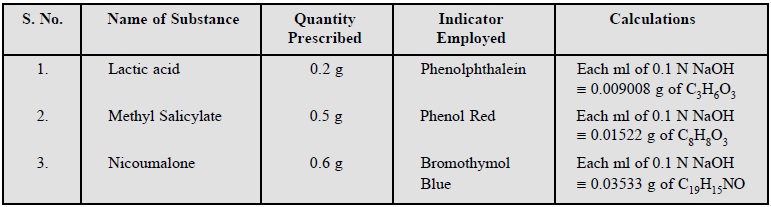
2.2. Cognate Assays
Lactic acid ; Methyl salicylate ; Nicoumalone.
Related Topics