Chapter: Essentials of Psychiatry: Antidepressants
Antidepressants: The Formulation of Treatment
The
Formulation of Treatment
Indications
All
antidepressants are indicated for the treatment of acute ma-jor depressive
episodes. Beyond the acute period, there is also evidence for the use of
antidepressants in the prevention of re-lapse and recurrence.
There are
a number of more minor forms of depression, many of which may also respond to
antidepressant medication. Best studied of these is dysthymic disorder.
Previously thought to be unresponsive to somatic therapy, a growing literature
at-tests to the responsiveness of this chronic, minor depressive disorder to a
variety of medications, including TCAs (Kocsis et al., 1985; Stewart et al.,
1993) and serotonin reuptake inhibi-tors (Hellerstein et al., 1993; Thase et al.,
1996; Ravindran et al., 1994;
Vanelle, 1997). As with major depression, there is no de-finitive data to
suggest that any one agent is more efficacious than the other. The bulk of data
suggests, instead, that any available agent used for major depression is likely
to be effective for these other disorders.
Other
minor depressive disorders include minor depres-sion and recurrent brief
depression. Though rigorous data is largely lacking in the treatment of these
disorders, they seem to show an at least modest response to antidepressant
medications.
Other Uses for Antidepressants
Although
we will describe the use of anti-depressants in the treatment of major
depression, they are also used to treat a number of other conditions (Orsulak
and Waller, 1989; Brotman, 1993). Some uses have gained general accept-ance
while other uses rely on moderate or preliminary evidence. A summary of various
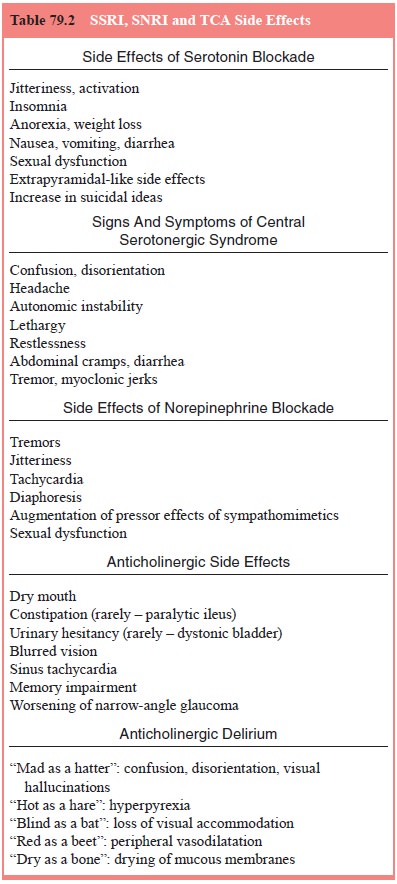
indications is presented in Table 79.2.
Selection of an Antidepressant
The
decision whether to treat depressive symptoms with phar-macotherapy requires an
assessment of both the need for in-tervention and the likelihood that treatment
will be successful. Assessing the need for intervention involves longitudinal
and cross-sectional factors. Assessing the likelihood that treatment will be
successful is somewhat more difficult, but may rely on clinical, demographical
and biological factors.
Assessing the Need for Intervention
This
involves assessing the likely result if pharmacological treat-ment is not
given. It is essential in making a useful risk–benefit assessment.
Longitudinal Factors The
physician should consider the course and
duration of previous episodes of depression. Such episodes can predict the
potential severity of the current episode, the likely time to recovery, and the
probability of a subsequent recurrence. The physician should also consider the
likely complications of depression for the individual patient, which may
include sub-stance abuse and suicide.
Cross-sectional Factors The
physician should consider the severity
of symptoms and the degree of functional impairment. Suicidal ideation is of
particular concern and needs rapid and in-tensive treatment. Such treatment
often includes hospitalization. Even with less pressing symptoms, but
significant occupational or social impairment, the risk–benefit ratio generally
still favors a trial of antidepressants, particularly now that safer and more
easily tolerated agents are available.
Selection of a Particular Agent Although,
as noted earlier, the various
antidepressants seem to have equal efficacy in the treat-ment of depression, a
given patient may respond preferentially to one, or a class of agents. Again,
cross-sectional and longitudinal factors should be taken into account.
Pharmacokinetic Concerns
First-generation Antidepressants The
pharmacokinetics of TCAs is complex.
This complexity is reflected in the diversity of half-lives reported, which
vary roughly from 10 to 40 hours. TCAs are primarily absorbed in the small
intestine. They are usually well absorbed, and reach peak plasma levels 2 to 6
hours
after
oral administration. Absorption can be affected by changes in gut motility. The
drugs are extensively metabolized in the liver on first pass through the portal
system. They are lipophilic, have a large volume of distribution and are highly
protein-bound (85–95%). TCAs are metabolized in the liver by hepatic
micro-somal enzymes, by demethylation, oxidation, or hydroxylation. They are
generally metabolized to active metabolites, and are excreted by the kidneys.
There is a large range of elimination half-lives among the antidepressants.
MAOIs are
also well absorbed from the gastrointestinal tract. Their metabolism, although
quite efficient (they have a half-life of 1 to 2 hours), is not well
understood. The short half-life of these compounds is not entirely relevant
however, as they bind irreversibly with MAO. Thus, the activity of these drugs
depends less on pharmacokinetics, and more on the synthesis of new MAO to
restore normal enzyme activity. This synthesis re-quires approximately 2 weeks.
Second-generation Antidepressants All of
the available serot-onin reuptake inhibitors are well absorbed, and not
generally af-fected by food administration. Sertraline is an exception to this
rule, and its blood level may be increased by food. All serotonin reuptake
inhibitors have large volumes of distribution and they are extensively
protein-bound. They are metabolized by hepatic microsomal enzymes and are
potent inhibitors of these enzymes (a fact which will be discussed later in
greater detail).
The only
serotonin reuptake inhibitor with an active me-tabolite is fluoxetine, whose
metabolite norfluoxetine has a half-life of 7 to 15 days. Thus, it may take
several months to achieve steady state with fluoxetine. This is considerably
longer than cita-lopram, which has a half-life of about 1.5 days, or sertraline
and paroxetine, which have half-lives of about a day.
As
previously discussed, there is no correlation between half-life and time to
onset. Drugs with shorter half-lives have an advantage in cases where rapid
elimination is desired (for exam-ple, in the case of an allergic reaction).
Drugs with a longer half-life may also have advantages: fluoxetine, for
example, has been successfully given in a once-weekly dosing during the
continu-ation phase of treatment (Burke et
al., 2000), and a once-weekly formulation of this drug is currently
available. All serotonin reuptake inhibitors are eliminated in the urine as
inactive me-tabolites. Both fluoxetine and paroxetine are capable of inhibiting
their own clearance at clinically relevant doses. As such, they have nonlinear
pharmacokinetics: changes in dose can produce proportionately large plasma
levels.
As with
most of the other antidepressants, bupropion un-dergoes extensive first pass
metabolism in the liver. Although the parent compound has a half-life of 10 to
12 hours, it has three me-tabolites that appear to be active. One, threohydrobupropion,
has a half-life of 35 hours and is relatively free in plasma (it is only 50%
protein-bound). There is considerable individual variability in the levels of
bupropion and its metabolites. Trazodone has a half-life that is relatively
short, having a range of 3 to 9 hours. Given this, and its apparent lack of
active metabolites, the plasma levels of trazodone can be quite variable during
a day. For this reason, the medication requires divided dosing.
Third-generation Antidepressants Venlafaxine
has a short half-life (4 hours);
however, it is available in an extended release formulation that allows
once-daily dosing. It appears to have a dual effect, in which at lower doses it
primarily acts on the serotonin transporter, and clinically significant
norepinephrine reuptake inhibition is not seen until higher doses are used (150
mg/day and above).
Nefazodone
has relatively low bioavailability, and a short half-life (2–8 hours), and thus
it is usually given in twice-daily doses. Mirtazapine has a half-life of 13 to
34 hours.
Related Topics