Chapter: Essentials of Psychiatry: Antidepressants
Antidepressants: Preparation of the Patient
Preparation
of the Patient
Side Effects of Antidepressants
General Concerns
Good
preparation and reassurance are essential. Side effects – even relatively
benign ones – are a major cause of treatment non-adherence. Drop-out rates
ranging from 7 to 44% have been reported in various studies of TCAs, and from 7
to 23% in studies of serotonin reuptake inhibitors (Cookson, 1993). Proper
educa-tion and reassurance about side effects can help reduce this rate. It
should help reassure the patient that many of the side effects di-minish with
time, or with an adjustment of dose. It may also help frame side effects in a
positive light, as they represent concrete evidence that the medication is
exerting its effect on the body.
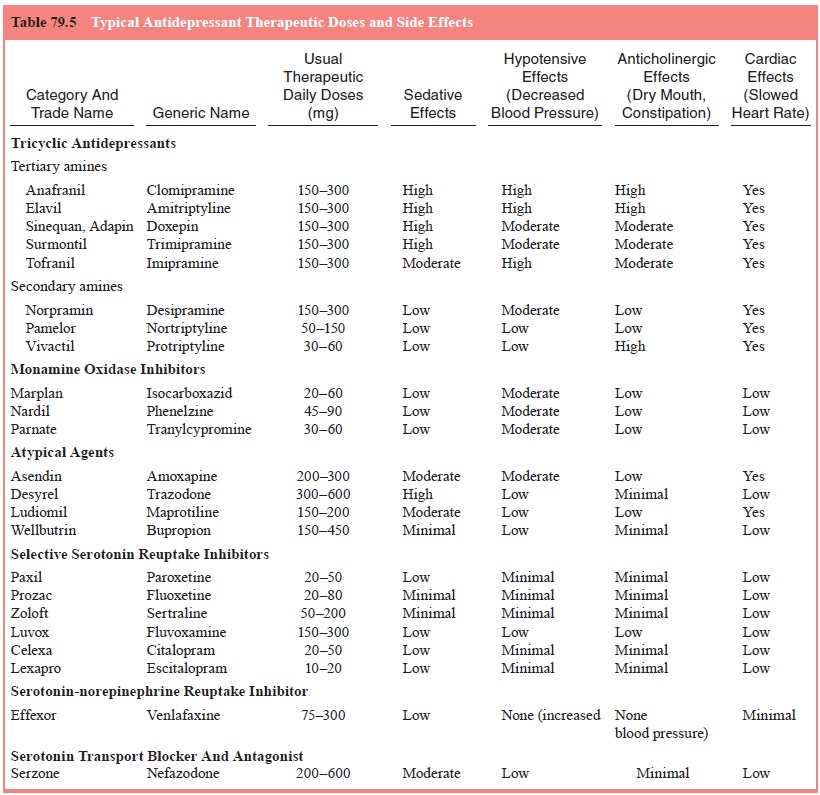
The best
approach may be to consider both frequency and clinical importance. That is,
one should discuss those side effects that are likely to occur, as well as
considering the rare but poten-tial dangerous or irreversible side effects that
should be discussed (Table 79.3 and Table 79.4).
Specific Side Effects
It is
useful to divide side effects into “predictable” and idiosyn-cratic effects.
Predictable side effects result from known pharma-cological actions of the
drug. Idiosyncratic side effects are not well understood. A number of authors
have written important, and very complete reviews of medication-related side
effects (e.g., Cookson, 1993; Nierenberg, 1992; Mir and Taylor, 1997;
Richelson, 2001); what is intended in the following paragraphs and figures is a
brief summary that incorporates data from those works.
Predictable Side Effects
These
side effects are the result of the action of the agent at vari-ous
neurotransmitters and enzyme sites. The major neurotrans-mitters affected by
antidepressants are as follows.
Muscarinic Acetylcholine Receptors
Blockade
of this receptor produces a variety of peripheral and central effects.
Gastrointestinal effects include decreased saliva-tion and decreased
peristalsis. Decreased salivation is the most common of these effects and can
cause drying of the mucous membranes. Such drying can exacerbate gum disease
and den-tal caries. Decreased peristalsis can cause constipation and, in the
extreme, paralytic ileus. Contraction of the bladder wall is inhibited, causing
urinary hesitancy and even urinary retention. In the case of TCAs, concomitant
sympathomimetic effects that cause constriction of the bladder neck and urethra
worsen this ef-fect on urination. Inhibition of the parasympathetically
mediated accommodation reflex, in which the ciliary body muscles nor-mally
contract to thicken the lens and focus near objects on the retina, results in
blurry vision and mydriasis. Such accommoda-tion paresis can occur without
other anticholinergic side effects. A more serious ocular effect is the
precipitation of acute narrow-angle glaucoma, through pupillary dilatation. The
iatrogenic pre-cipitation of narrow-angle glaucoma through antidepressant use
is quite rare. Anticholinergic cardiac effects include decreased vagal tone
that can cause tachycardia. Central nervous effects in-clude impaired memory
and cognition. In severe cases, such cog-nitive impairment can reach the point
of a delirium. Central anti-cholinergic effects can also worsen existing
tardive dyskinesia.
These
effects are usually dose-related, and are worse in people with preexisting
defects. For example, the cardiac effects are of most concern in those with
preexisting cardiac defects, and urinary blockade generally occurs only in the
presence of prostatic hypertrophy. These side effects are also more common in
patients taking other anticholinergic medications, which is a common feature of
many over-the-counter preparations.
MAOIs
have little direct effect on receptors, and their side effects relate to
enzymatic inhibition, thus they are not included in this section.
Histamine
Blockade
of the histamine H1-receptor is typically associated with sedation.
Histamine blockade may also cause orthostatic hypo-tension and weight gain. It
can impair psychomotor coordination and cause falls in the elderly. Cognitive
impairment can occur as well. H2-receptor blockade causes decreased
gastric acid produc-tion. This is the mechanism of many anti-ulcer medications.
Norepinephrine
Synaptic
increases in norepinephrine, through either inhibition of norepinephrine
reuptake or decrease in MAO degradation, cause sympathomimetic effects.
Increases in norepinephrine can cause anxiety, tremors, diaphoresis and
tachycardia. This tachycardia can potentiate anticholinergic cardiac effects.
As noted, sym-pathomimetic effects on the bladder neck and urethra can
poten-tiate the anticholinergic inhibition of normal urinary function.
Receptor Blockade
Blockade
of alpha-1-receptors occurs as a chronic effect through both
downregulation and desensitization of the beta- and alpha-2-receptors.
Blockade of the noradrenergic alpha-1-receptor is responsible for
postural hypotension. In the elderly or medically ill, this postural
hypotension can be significant, and lead to diz-ziness or falls. It may also be
responsible for ejaculatory delay or impotence. Other potential effects include
reflex tachycardia and memory dysfunction.
Serotonin
Potentiation
of serotonin can cause anorexia, nausea, vomiting, diarrhea, “jitteriness” and
anxiety. Akathisia, a syndrome of mo-tor restlessness usually associated with
antipsychotics, may result from either the general effect of serotonin
potentiation or the direct effects on the basal ganglia. The latter hypothesis
is supported by the fact that the serotonin reuptake inhibitors – fluoxetine
(Steur, 1993), sertraline (Shihabuddin and Rapport, 1994) and paroxet-ine
(Choo, 1993) – have all been reported to cause or exacerbate extrapyramidal
reactions. Sedation, which has been reported with all serotonin reuptake
inhibitors, appears to be a primary serotonin effect (Cookson, 1993). Insomnia,
however, is more common at higher doses, particularly with fluoxetine. A number
of sexual side effects have been attributed to serotonin reuptake blockade,
including anorgasmia, ejaculatory difficulties and even spontaneous orgasms
associated with yawning (Modell, 1989).
Receptor Antagonism
Blockade
of the 5-HT2-receptors may result in hypotension and ejaculatory
disturbances. Antagonism of serotonin receptors may also be responsible for
weight gain and carbohydrate craving.
Dopamine
Increases
in dopamine resulting from reuptake blockade can have an antiparkinsonian
effect. It can also cause psychomotor activa-tion and aggravation of psychosis.
Receptor Antagonism
The
blockade of dopamine receptors can result in extrapyrami-dal symptoms. These
symptoms include cogwheel-type rigidity, tremor, dyskinesia, masked facies and
acute dystonia. Prolonged dopamine blockade appears to be responsible for
tardive dyski-nesia. Dopamine receptor blockade has also been associated with
endocrine changes and sexual dysfunction.
Monoamine Oxidase
MAO is
the main enzyme responsible for the metabolism of monoamines. There are two
main types of MAOs, identified as types A and B. Type A is selective for
serotonin and norepine-phrine, and accounts for 80% of the MAO in the brain.
Type B selectively deaminates phenylethylamine. Both forms oxidize dopamine and
tyramine.
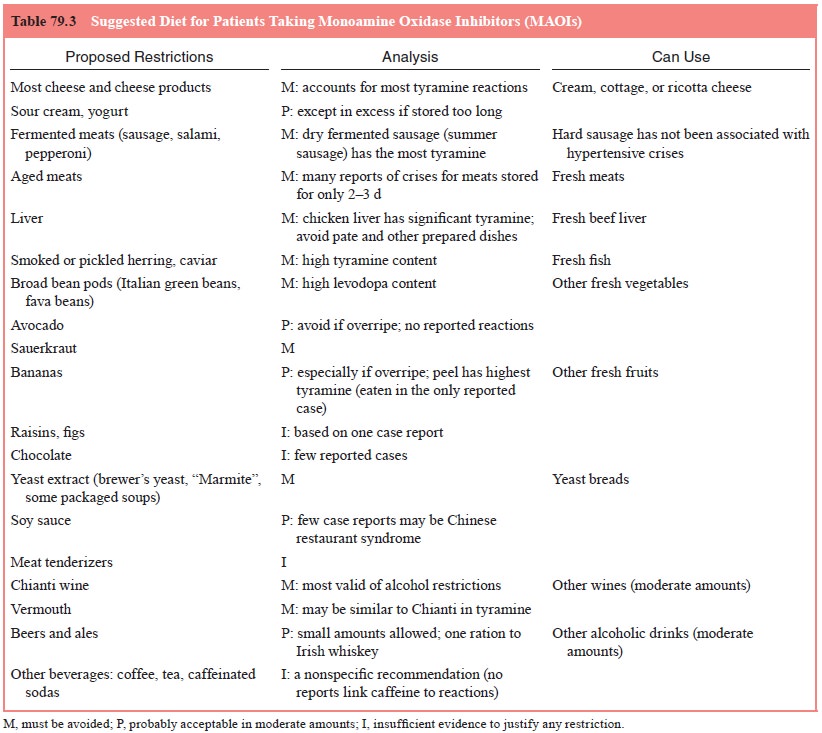
Dietary Restrictions The
dietary restrictions required when us-ing MAOIs represent the major limitation
to widespread use of these effective antidepressants. Nonselective inhibition
of MAO prevents the normal hepatic metabolism of tyramine containing foods or
sympathomimetic agents. The increased level of tyramine in the circulation
stimulates the release of norepinephrine from sympathetic terminals. This
sudden increase in norepinephrine is the basis for the “tyramine–cheese”
reaction, so named because cheese is the most common source of the tyramine
that causes this reaction. In fact, other pressor amines, such as levodopa, can
also cause the reaction, but tyramine – a natural product of food fermentation
and bacterial decarboxylation – is the most common in foods. The result of a
tyramine–cheese reaction can be a hy-pertensive crisis. Thus, patients should
be well educated as to the foods that must be avoided while using MAOIs. In the
past, there has been a tendency towards conservative dietary restrictions,
often based on single case reports or indirect analogies. More research and
experience have suggested that not all the foods commonly restricted are
equally likely to precipitate a reaction (McCabe and Tsuang, 1982). Better
compliance is likely if a more reasonable diet is prescribed (as suggested in
Table 79.5).
Despite
the best of preparation, some patients may err and suffer a hypertensive
crisis. This is often experienced as a se-vere, pulsating, occipital headache
that then generalizes. It may be alleviated with 10 mg of nifedipine, either
oral or sublingual (Golwyn and Sevlie, 1993).
MAOIs can
cause an increase in standing systolic blood pressure, absent of tyramine
containing foods or sympathomi-metics. Generally, this effect is not clinically
significant; however, serious unprovoked hypertensive episodes have been
reported
(Lavin et al., 1993), and blood pressure should
be monitored for 1 to 2 hours after beginning or increasing an MAOI.
Hypoten-sion is also a reported effect of MAO, but the mechanism is not known.
MAO inhibition can also cause sedation or overstimula-tion. Once again, the
mechanism of this is not well understood.
Membrane Stabilizing Activity
TCAs have
effects on cardiac conduction that are independent of anticholinergic or
noradrenergic effects. Destabilization of the cardiac membrane can cause
dysrhythmia and asystole, particu-larly in overdose.
Other Effects
Allergic Reactions
Allergic
reactions can occur with any of these agents. Effects in-clude dermatological
(rashes, urticaria, photosensitivity, Stevens Johnson syndrome) and
hematological (agranulocytosis) sensi-tivities. Several case reports have
described a photosensitivity reaction apparently caused by desipramine that
results in a blue– gray pigmentation (Narurkar et al., 1993; Steele and Ashby, 1993). Fluoxetine has been
associated with bleeding, inflammation (Gunzberger and Martinez, 1992), and,
most seriously, a fatal systemic vasculitis. It should be stopped if a rash
develops.
In most
cases of allergic reactions, the primary treatment is to stop the agent. In one
report, granulocyte colony stimulating factor was used successfully to treat
severe chlomipramine-as-sociated agranulocytosis (Hunt and Resnick, 1993).
Liver Effects
Abnormal
liver function tests have been associated with a number of antidepressants,
which can be independent of dose. The risk for such effects may be worsened by
chronic alcohol or anticonvulsant use.
Seizures
A
preexisting seizure disorder increases an antidepressant’s likelihood of
precipitating a seizure. Other predisposing factors include a family history of
a seizure; an abnormal pretreatment electroencephalogram; brain damage;
previous electroconvul-sive treatment; abuse or withdrawal from sedatives;
alcohol, or cocaine; and concurrent use of CNS-activating medications
(Rosenstein et al., 1993). Seizures
may be more likely to occur early in treatment, or after a large escalation in
dose.
The risk
of seizure with TCAs is usually reported as 0.1–1.1%. In unselected populations
the risk may be as high as 2 to 2.5% (Davidson 1989). Serotonin reuptake
inhibitors appear to have a lower incidence of seizures. Bupropion has a high
rate of seizures in patients with a preexisting seizure history, and in
patients with bulimia. In patients without these predisposing factors, the risk
appears to be about 0.4%; thus, it may have a two- to fourfold risk of seizures
compared with other antidepressants. Bupropion’s effect on the sei-zure
threshold has never been directly compared with other antidepressants.
Precipitation of Mania
Antidepressants
have been associated with the precipitation of mania, and rapid cycling bipolar
disorder. This appears to be most common in patients with a preexisting history
of mania and in unipolar depression the rate of antidepressant-induced mania is
very low (,1%). The problem has been most frequently re-ported
in TCAs, but has been seen in SSRIs as well (Cookson, 1993). A similar problem
has been found with newer agents, in-cluding nefazodone and venlafaxine, though
the data for this is more limited. Bupropion may have a lower incidence of
mania (Shopsin, 1983).
Sexual Dysfunction
A variety
of sexual side effects can be caused by antidepressants, and they can affect
all aspects of sexual response. Thus, anti-depressants can decrease libido,
increase impotence and anor-gasmia, and cause delayed or retrograde ejaculation
(Segraves, 1992).
SSRIs
have a high incidence of delayed orgasm or anor-gasmia. Sexual side effects can
occur at even low therapeutic doses, and dose reduction may not be possible. A
change of agent may be the only alternative. Bupropion appears to have the
lowest incidence of sexual side effects among the antide-pressants, often not
differing from placebo in studies of sexual functioning.
Trazodone
has been associated with penile priapism; the risk is around 1 in 6000 to 8000
men. Although rare, it is nota-ble that a third of these cases required
surgical intervention, and some resulted in permanent impairment. Clitoral
priapism has been reported as well (Pescatori et al., 1993).
Occasionally
antidepressants can enhance sexual func-tion. This is usually due to the
alleviation of depression; however, there have been case reports of improved
libido or potency after initiation of an antidepressant, which occurred
independent of any antidepressant effect (Smith and Levitte, 1993).
Miscellaneous
Fine
tremors have been noticed with both TCAs and serotonin reuptake inhibitors. The
syndrome of inappropriate secretion of antidiuretic hormone has been seen with
fluoxetine, as well as with a number of other antidepressants.
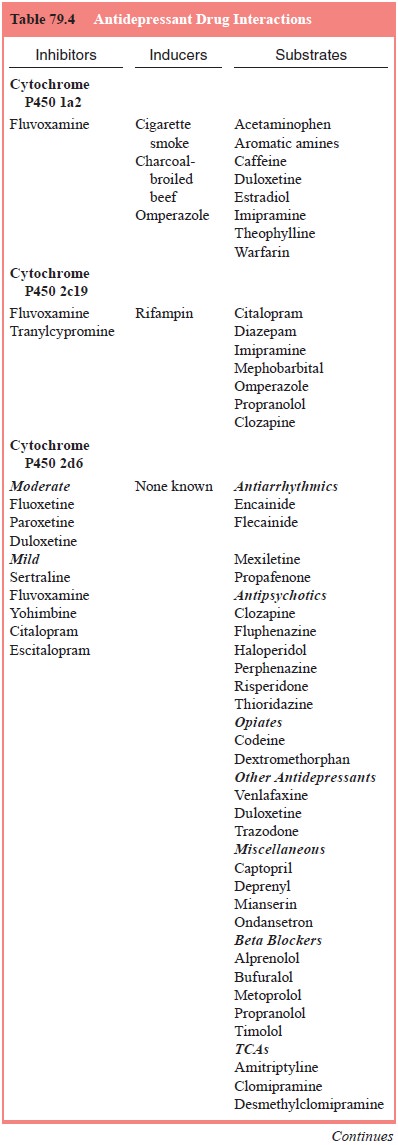
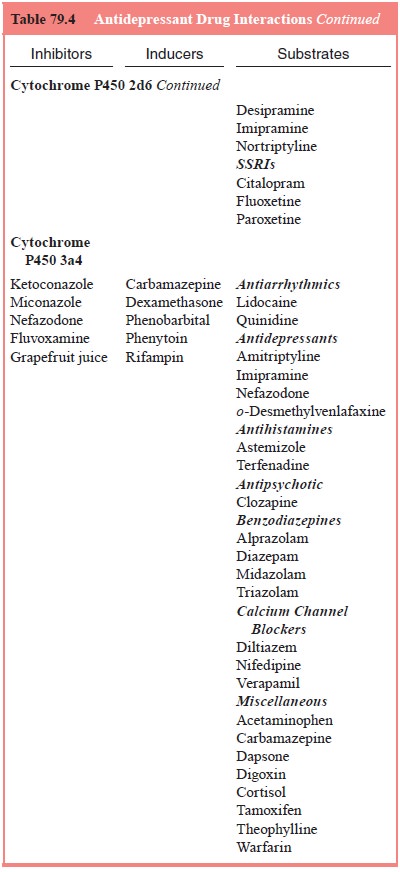
Drug Interaction Effects
Drug
interactions are summarized in Table 79.4.
First-generation Antidepressants
Tricyclic Antidepressants
As with
any combination therapy, the side effects described previously can be additive
with other similar drugs. Most problematical are the anticholinergic effects of
the TCAs. Such cholinergic – particularly muscarinic – blockade is a property
shared by many other medications, including numerous over-the-counter
preparations. The general sedative properties of these medications can also
augment any soporific. The slow-ing of cardiac conduction can also potentiate other
medications that produce similar effects, such as type IA antiarrhythmics and
anticholinergic medications. Adrenergic receptor blockade can worsen the
orthostatic hypotension caused by other medi-cations, including vasodilators
and low-potency antipsychotic medications.
Absorption
of TCAs can be inhibited by cholestyramine, which therefore must be given at
different time intervals than the antidepressants. TCA levels can be raised by
substances that inhibit enzyme activity, and lowered by substances that induce
it. Specific substances reported to increase TCA levels include fluoxetine,
antipsychotic medications, methylphenidate and ci-metidine. In a controlled
trial, methylphenidate was combined with desipramine to treat attention
deficits and depression in children. Enzyme “inducers” that can lower tricyclic
agent lev-els include phenobarbital and carbamazepine. The nicotine from
cigarettes can also induce enzyme activity.
Guanethidine
is contraindicated with TCAs, as it relies on neuronal reuptake for its
antihypertensive effect. Clonidine,
presynaptic alpha-2-receptor
noradrenergic agonist, is also contraindicated, as it works in an antithetical
fashion to tricyclic medications.
Monoamine Oxidase Inhibitors
As with
the dietary proscriptions, any medication that increases tyramine can
precipitate a hypertensive crisis. Such medications include numerous
over-the-counter preparations for coughs, colds and allergies. The same rule
applies to sympathomimetic drugs (such as epinephrine and amphetamines) and
dopaminer-gic drugs (such as anti-Parkinsonian medications).
The
combination of MAOIs and narcotics – particularly meperidine – may cause a
fatal interaction. The reaction can vary from symptoms of agitation and
hyperpyrexia to cardiovas-cular collapse, coma and death. The mechanism of this
reaction is poorly understood. A similar reaction has also been reported when
propoxyphene, diphenoxylate hydrochloride and atropine are used with MAOIs.
The
combination of an MAOI with a potentiator of serot-onin (such as a serotonin
reuptake inhibitor) can cause the serot-onin syndrome.
Similar
to the dietary restrictions, some of the drug re-strictions associated with
MAOIs are based on few actual data. Best established are the restrictions
against the combination of MAOIs with amines, meperidine, dextromethorphan,
hypogly-cemic agents, l-dopa, reserpine, tetrabenazine and tryptophan. TCAs are
frequently included on this list as causing a “central excitatory syndrome” in
combination with MAOIs, although the two have been combined safely. Blackwell
(1991) published a comprehensive review of MAOI drug interactions.
Second-generation Antidepressants
Serotonin Reuptake Inhibitors
Serotonin
reuptake inhibitors are potent inhibitors of the CYP2D6 pathway, and the drug–drug
interactions that can result from this have been the subject of a number of
books and articles.
Serotonin
reuptake inhibitors can slow the metabolism of any drug that is also
metabolized by the same cytochrome P450 pathway. Such drugs include TCAs,
carbamazepine, phenothi-azines, butyrophenones, opiates, diazepam, alprazolam,
vera-pamil, diltiazem, cimetidine and bupropion. Paroxetine appears to be the
most potent inhibitor of this metabolic pathway, with fluoxetine also showing
high potency. Sertraline is a somewhat less potent inhibitor.
These
pharmacokinetic interactions are best managed with dosage adjustment.
Fluoxetine, for example, can be safely used with tricyclic medications if TCA
blood levels and, possibly, elec-trocardiograms are monitored. In the case of
bupropion, this rela-tive increase in the blood level can increase the risk for
seizures.
Particular
caution should be used when a patient using multiple medications starts a
serotonin reuptake inhibitor, as the interactions with other drugs can cause
dangerous increases in levels. For example, in the cardiac patient, levels of
warfa-rin should be monitored as fluoxetine has been reported to raise these
levels (Woolfrey et al., 1993).
Several case reports exist of increased antiarrhythmic levels after
introduction of fluoxetine, which resulted in potential serious
bradyarrhythmias.
Fluoxetine
has also been reported to raise lithium levels. The mechanism for this is not
clear, as lithium is primarily ex-creted through the kidneys.
Serotonin Syndrome
Of
particular concern is the serotonin syndrome. This syndrome occurs when a
serotonin reuptake inhibitor is combined with an-other drug that can potentiate
serotonin, such as MAOIs, penta-zocine and l-tryptophan. It has also been reported
with the ad-junctive use of less obvious serotonergic drugs, such as lithium
(Muly et al., 1993) and carbamazepine
(Dursun et al., 1993). This creates a
toxic effect with symptoms of abdominal pain, diarrhea, diaphoresis,
hyperpyrexia, tachycardia, hypertension, myoclonus, irritability, agitation,
epileptic seizures and delirium. In its severest form, it can result in coma,
cardiovascular shock and death. For this reason, a clearance period is required
before switching between a serotonin reuptake inhibitor and an MAOI. Switching
from fluoxetine to an MAOI is particularly difficult, given fluoxetine’s long
clearance time – about 6 weeks. Clear-ance is considerably more rapid for
sertraline or paroxetine, and a 2-week “wash-out” period is advised when
changing from one of these agents to an MAOI. Occasionally, case reports have
sug-gested that some patients tolerate a quicker switch; however, a full
waiting period remains the most prudent course, as several deaths have occurred
after an MAOI was begun too soon after fluoxetine was discontinued (Beasley et al., 1993).
Other Second-generation Antidepressants
Few
reports exist of interactions with other drugs and trazodone, although
trazodone may increase levels of digoxin, phenytoin and possibly warfarin.
Bupropion causes few drug–drug interactions. The main interactions reported
have occurred when bupropion is combined with another dopaminergic agent. For
example, when bu-propion was used with l-dopa, the combination caused
excitement, restlessness, nausea, vomiting and tremor (Goetz et al., 1984).
Third-generation Antidepressants
Venlafaxine
does not substantially inhibit the CYP enzyme, and is not highly protein-bound,
thus it tends to have few clini-cally significant drug–drug interactions.
Nefazodone is highly protein-bound, and has several active metabolites. It is
also a strong inhibitor of CYP3A4, and affects other drugs also me-tabolized by
that pathway; however, it has little affinity for the CYP2D6 enzyme.
Mirtazapine is highly protein-bound as well, but appears only weakly to affect
the cytochrome enzymes.
Related Topics