Chapter: Microbiology and Immunology: Antimicrobial Agents Therapy and Resistance
Antibiotic Sensitivity Testing
Antibiotic Sensitivity Testing
Antibiotic sensitivity testing is carried out to determine the
appropriate antibiotic agent to be used for a particular bacte-rial strain
isolated from clinical specimens. Antibiotic sensitiv-ity testing can be
carried out by two broad methods, as follows:
a)
Disc diffusion tests
b)
Dilution tests
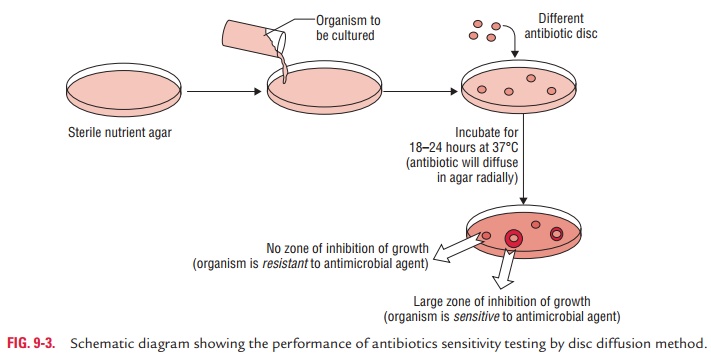
Disc Diffusion Tests
Disc diffusion tests are the most commonly used methods in a
laboratory to determine susceptibility of bacteria isolates to antibiotics. In
this method, as the name suggests, discs impregnated with known concentrations
of antibiotics are placed on agar plate that has been inoculated with a culture
of the bacterium to be tested. The plate is incubated at 37°C for 18–24 hours.
After diffusion, the concentration of antibi-otic usually remains higher near
the site of antibiotic disc, but decreases with distance. Susceptibility to the
particular antibi-otic is determined by measuring the zone of inhibition of
bacte-rial growth around the disc (Fig. 9-3).
◗ Selection of media
The medium that supports both test and control strains is selected
for carrying out antibiotic susceptibility testing of the bacteria. For
example, Mueller–Hinton agar is used for testing Gram-negative bacilli and Staphylococcus spp., blood agar for Streptococcus spp. and Enterococcus spp. species, chocolate
agarfor Haemophilus influenzae, and
Wellcotest medium for sulfon-amides and cotrimoxazole.
The medium is prepared by pouring onto the flat horizon-tal surface
of Petri dishes of 100 mm to a depth of 4 mm. The pH of the medium is
maintained at 7.2–7.4. More alkaline pH increases the activity of
tetracyclines, novobiocin, and fusidic acid, whereas an acidic pH reduced the
activity of aminogly-cosides and macrolides, such as erythromycin. The plates
after preparation may be stored at 4°C for up to 1 week.
◗ Preparation of the inoculum
For testing antibiotic sensitivity, the
bacteria are first isolated in pure culture on a solid medium. At least three
to four morpho-logically similar colonies of the bacteria to be tested are
touched and inoculated into appropriate broth and incubated at 37°C for 4–6
hours. The density of bacterial suspension in the broth is adjusted to 1.5 108 cfu/mL
by comparing its turbidity with that of 0.5 McFarland opacity standard tube.
The broth is inoculated on the medium by streaking with sterile swabs. A
sterile cotton swab is dipped into the broth and excess broth is removed by
rota-tion of the swab against the sides of the tube above the fluid level.
◗ Antibiotic discs
Only the clinically relevant antibiotics are tested in antibiotic
susceptibility tests. Antibiotic discs (6-mm filter paper discs) can be
prepared from pure antimicrobial agents in laborato-ries or can be obtained
commercially. The discs are applied with sterile forceps, a sharp needle, or a
dispenser onto the surface of the medium, streaked with test strains, and the
reading is reported after incubating the plate for 18–24 hours at 37°C
aerobically.
◗ Types of disc diffusion tests
Disc diffusion tests are of the following types:
a)
Kirby–Bauer disc diffusion method
b)
Stokes disc diffusion method
c)
Primary disc diffusion test
Kirby–Bauer disc diffusion
method: Kirby–Bauer discdiffusion method is the most common method used
routinely for determination of antibiotic sensitivity of bacteria isolated from
clinical specimens. In this method, both the test strains and the control
strains are tested in separate plates.
The test is performed by inoculating the test organism in a
suitable broth solution, followed by incubation at 37°C for 2–4 hours. Then 0.1
mL of the broth is inoculated on the surface of the agar medium by streaking
with a sterile swab. In this method, either nutrient agar or Mueller–Hinton
agar in Petri dishes is used. The inoculated medium is incubated overnight at
37°C. The susceptibility of drug is determined from the zones of inhibition of
bacterial growth surrounding the anti-biotic discs. The diameters of the zone
of inhibition are calcu-lated with vernier calipers or a thin transparent
millimeter scale to the nearest millimeter. The point of abrupt diminution of
the zone is considered as the zone edge. A maximum of six anti-biotic discs are
tested in a Petri dish of 85 mm size (Fig. 9-4).
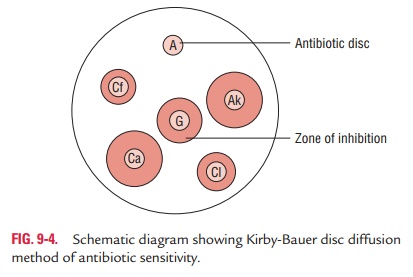
Interpretation of the zone size is done as per the interpreta-tion
chart. Depending on the zone size, bacteria can be consid-ered sensitive,
intermediate or resistant to antibiotics.
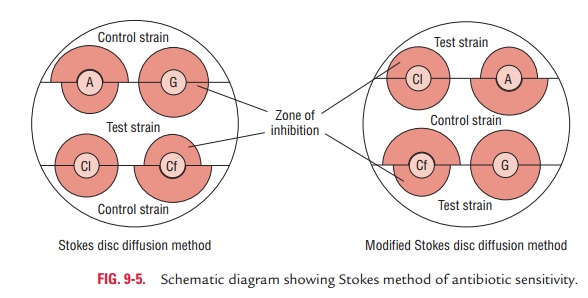
Stokes method: This is a disc diffusion
method, which makesuse of inbuilt controls against many variables. In this method,
the Petri dish containing the Mueller–Hinton agar is divided horizontally into
three parts. The test strain is inoculated in the central area and the control
strains on the upper and lower third of the plate. In modified Stokes method,
control strain is inoculated in the central part but test strains are
inoculated on the upper and lower third of the plate. The plates are incubated
at 37°C and observed for zones of bacterial inhibition around the discs (Fig.
9-5). A maximum of six antibiotic discs can be applied on a 100 mm Petri dish.
Reporting of the result is
carried out by comparing the zones of inhibition of test and control bacteria.
The zone sizes are measured from the edge of the disc to edge of the zone. It
is interpreted as follows:
·
Sensitive (S): The zone of test bacterium is
equal to or morethan that of control strain. The differences between the zone
sizes of control and test strains should not be more than 3 mm if the zone size
of the test bacterium is smaller than that of control.
·
Intermediate (I): The zone size of the test
bacterium shouldbe at least 2 mm, and the differences between the zone of test
and control strain should be at least 3 mm.
·
Resistant (R): The zone size of the test
bacterium is 2 mmor less.
Interpretation of disc diffusion tests:Results of disc diffusiontests, such as Kirby–Bauer and Stokes
method, are interpreted as follows:
·
Sensitive (S): Infection treatable by the
normal dosage ofthe antibiotic.
·
Intermediate (I): Infection may respond to
higher dosage.
·
Resistant (R): Unlikely to respond to usual
dosage of theantibiotics.
However, for certain bacteria and antibiotic discs, the following
may be kept in mind while interpreting results of disc diffusion tests:
a)
Proteus
mirabilis, Proteus
vulgaris, and other bacteria producingswarming produce a thin film on agar
surface often extend-ing into the zones of inhibition. In such situations, the
zones of swarming should be ignored and the outer clear margin should be
measured to determine the zones of inhibition.
b)
Many strains of MRSA grow very slowly in the presence of
methicillin. They produce growth within the zone of inhi-bition on incubation
for more than 48 hours. This prob-lem can be overcome by incubating the
bacteria at 30°C or by using 5% salt agar and incubating at 37°C.
c)
Penicillinase producing strains of Staphylococcus often fail to secrete enough enzymes to neutralize
penicillin close to the antibiotic disc. In such situation, it may show a zone
of inhibition, but with the presence of large colonies at the edge of the zone
and without any gradual fading away of the growth of the bacteria toward the
disc. In such condi-tion, the zones of inhibition should not be considered, and
it should be reported resistant irrespective of the zone size.
d)
Trimethoprim and sulfamethoxazole should be tested separately to
know whether the bacterium is sensitive to both or only to one of these. These
should never be tested in combination, the way these two drugs are used in
com-bination in clinical practice.
Dilution Tests
Dilution tests are performed to determine the minimum inhib-itory
concentration (MIC) of an antimicrobial agent. MIC is defined as the lowest
concentration of an antimicrobial agent that inhibits the growth of organisms.
Estimation of the MIC is useful to:
·
Regulate the therapeutic dose of the antibiotic accurately in the
treatment of many life-threatening situations, such as bacterial endocarditis.
·
Test antimicrobial sensitivity patterns of slow-growing bacteria,
such as M. tuberculosis.
Following methods are carried out to determine the MIC:
a)
Broth dilution method
b)
Agar dilution method
c)
Epsilometer test (E-test)
◗ Broth dilution method
The broth dilution method is a quantitative method for deter-mining
the MIC of an antimicrobial agent that inhibits the growth of organisms in vitro. In this method, the
antimicrobial agent is serially diluted in Mueller–Hinton broth by doubling
dilution in tubes and then a standard suspension of the broth culture of test
organism is added to each of the antibiotic dilu-tions and control tube. This
is mixed gently and incubated at 37°C for 16–18 hours. An organism of known
susceptibility is included as a control. The MIC is recorded by noting the
lowest concentration of the drug at which there is no visible growth as
demonstrated by the lack of turbidity in the tube. The main advantage of this
method is that this is a simple procedure for testing a small number of isolates.
The added advantage is that using the same tube, the minimum bactericidal
concentration (MBC) of the bacteria can be determined.
The MBC is determined by subculturing from each tube, showing no
growth on a nutrient agar without any antibiotics. Subcultures are made from
each tube showing no growth into the nutrient agar plates without any
antibiotics. The plates are examined for growth, if any, after incubation
overnight at 37°C. The tube containing the lowest concentration of the drug
that fails to show any growth on subculture plate is considered as the MBC of
the antibiotic for that strain. Broth dilution may be of two
types—macrodilution and microdilution. Broth microdilu-tion is done using
microtiter plates and is considered the “gold standard.”
◗ Agar dilution method
Agar dilution method is a quantitative method for determining the
MIC of antimicrobial agent against the test organism.
Mueller–Hinton agar is used
in this method. Serial dilution of the antibiotic are made in agar and poured
onto Petri dishes. Dilutions are made in distilled water and added to the agar
that has been melted and cooled to not more than 60°C. One control plate is
inoculated without antibiotics. Organism to be tested is inoculated and
incubated overnight at 37°C. Plates are examined for presence or absence of
growth of the bacte-ria. The concentration at which bacterial growth is
completely inhibited is considered as the MIC of the antibiotic.
The organisms are reported sensitive, intermediate, or resis-tant
by comparing the test MIC values with that given in CLSI guidelines. The main
advantage of the method is that a num-ber of organisms can be tested
simultaneously on each plate containing an antibiotic solution.
◗
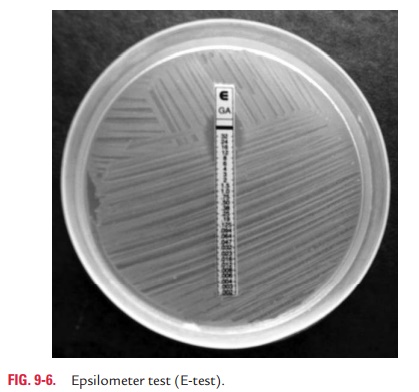
Epsilometer test (E test)
Epsilometer test (E test), based on the principle of disc
diffu-sion, is an automated system for measuring MIC of a bacterial isolate. In
this method, an absorbent plastic strip with a con-tinuous gradient of
antibiotic is immobilized on one side. MIC interpretative scale corresponding
to 15 twofold MIC dilu-tions is used on the other side. The strip is placed on
the agar plate inoculated with the test organism with the MIC scale fac-ing
toward the opening side of the plate. An elliptical zone of growth inhibition
is seen around the strip after incubation at 37°C overnight. The MIC is read
from the scale at the intersec-tion of the zone with the strip. The end point
is always read at complete inhibition of all growth including hazes and
isolated colonies. E test is a very useful test for easy interpretation of the
MIC of an antibiotic (Fig. 9-6).
Related Topics