Chapter: Modern Pharmacology with Clinical Applications: Hypothalamic and Pituitary Gland Hormones
Anterior Pituitary Hormones
ANTERIOR
PITUITARY HORMONES
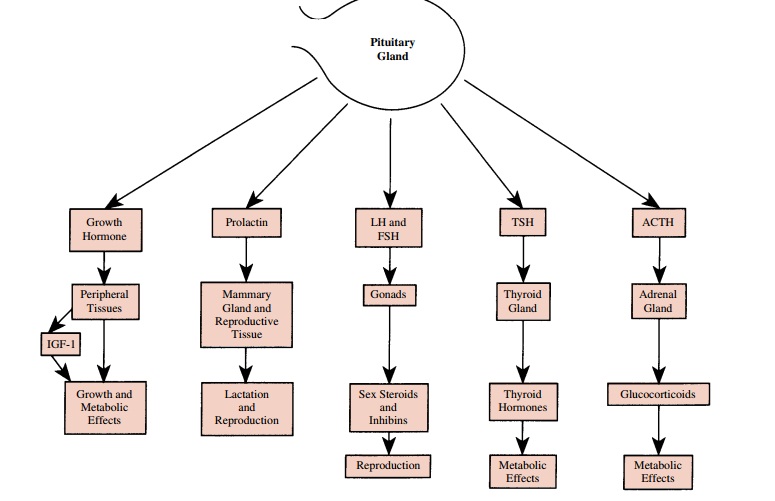
Six major hormones are
secreted by the adenohypoph-ysis, or anterior pituitary gland (Fig. 59.1).
Cells in the anterior pituitary gland also secrete small amounts of a variety
of other proteins, including renin, angiotensino-gen, sulfated proteins,
fibroblast growth factor, and other mitogenic factors. The physiological
significance of these other secretory products is not known, but they may
participate in autocrine regulation of the gland.
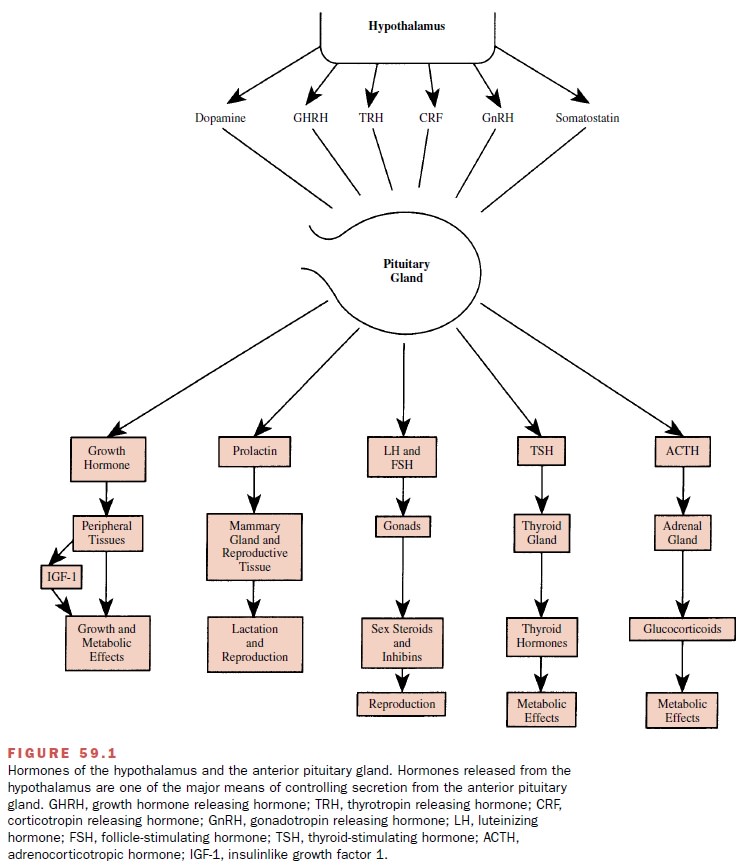
The secretion of anterior
pituitary hormones is con-trolled in part by hypothalamic regulatory factors
that are stored in the hypothalamus and are released into the adenohypophyseal
portal vasculature. Hypothalamic regulatory factors so far identified are
peptides with the exception of dopamine. Secretion of anterior pituitary
hormones is also controlled by factors produced more distally that circulate in
the blood. Predominant control of hormone production may be relatively simple,
as with thyroid-stimulating hormone (TSH), the production of which is primarily
stimulated by thyrotropin-releasing hormone (TRH) and inhibited by thyroid
hormones, or it may be complex, as with prolactin, the production of which is
affected by many neurotransmitters and hor-mones.
All anterior pituitary
hormones are released into the bloodstream in a pulsatile manner; the secretion
of many also varies with time of day or physiological con-ditions, such as
exercise or sleep. At least part of the pul-satility of anterior hormone
secretion is caused by pul-satile secretion of hypothalamic regulatory
hormones. Understanding the rhythms that control hormone secre-tion has led to
better uses of hormones in therapy.
Growth Hormone
Growth hormone, or
somatotropin, is a protein that stimulates linear body growth in children and
regulates cellular metabolism in both adults and children. Growth hormone
stimulates lipolysis, enhances production of free fatty acids, elevates blood
glucose, and promotes positive nitrogen balance. Many of its anabolic actions
are mediated by enhanced production of an insulinlike growth factor (IGF-1), a
protein produced in many tis-sues in response to growth hormone.
The episodic release of
growth hormone is the most pronounced among the pituitary hormones. Serum
lev-els between bursts of release are usually low (< 5 ng/mL) and increase
more than 10-fold when release is elevated. The marked variation in serum levels
is in part the result of strong controls in opposite directions by the
hypothalamic hormones, growth hormone–releasing hormone (GHRH), and
somatostatin. Circulating fac-tors, such as IGF-1 and ghrelin, a peptide
produced in large amounts in neuroendocrine cells of the stomach, also affect
growth hormone secretion. Growth hormone is released during sleep, with maximum
release occur-ring an hour after the onset of sleep. Growth hormone is also
released after exercise, by hypoglycemia, and in response to arginine and
levodopa.
Growth Hormone Deficiency
Growth hormone deficiency in
children results in short stature and in adults increases fat mass and reduces
mus-cle mass, energy, and bone density. Measurements of serum growth hormone
levels are used for diagnosis of deficiency, but random measurements are not
useful, be-cause normal episodic release results in large variations in growth
hormone levels. Growth hormone deficiency is most convincingly demonstrated by
lack of response to provocative stimuli, such as administration of insulin,
levodopa, or arginine. Recently a combination of GHRH and ghrelin have been
used and have given large responses in normal subjects. Deficiencies are
corrected by giving human growth hormone. Growth hormone is also sometimes
given to individuals who are not growth hormone–deficient; it is used to
increase the height of girls with Turner’s syndrome and in certain conditions
to counteract the wasting that may occur in AIDS.
In the past human growth
hormone was prepared from human pituitary glands, but this source was
dis-continued after people who had received treatment contracted
Creutzfeldt-Jakob disease. Now two forms of recombinant human growth hormone
are available: somatropin (Humatrope
and others), which has the same amino acid sequence as pituitary-derived growth
hormone, and somatrem (Protropin),
which has an N-terminal methionine
that the pituitary form does not. Subcutaneous injections each evening, which
mimic the natural surge that occurs at the start of sleep, are the usual
regimen. Stimulation of growth in children is most effective when treatment
begins early.
Growth Hormone Excess
Acromegaly results from
chronic secretion of excess growth hormone, usually as a result of pituitary
ade-noma. Long bones will not grow in adults because the epiphyses are closed,
but bones of the extremities (hands, feet, jaw, and nose) will enlarge. The
skin and soft tissues thicken, and the viscera enlarge. Excessive growth
hormone secretion is demonstrated by elevated serum levels of growth hormone
after glucose adminis-tration, since glucose is less effective in inhibiting growth
hormone secretion in acromegalics than it is in normal subjects. In addition,
serum IGF-1 levels are el-evated in acromegalics.
The primary treatment of
acromegaly is surgery. Pharmacotherapy is used when surgical treatment is not
successful. Two dopamine agonists , bromocriptine and cabergoline, are
sometimes effec-tive; they are taken orally. Although dopamine stimu-lates
growth hormone release in normal individuals, it inhibits growth hormone
release in up to 50% of acromegalics. The somatostatin analogue octreotide is
usually more effective, and now that a long-acting form is available that
requires only monthly injections, it is the preferred treatment. Another
possible growth hor-mone antagonist, pegvisomant, is being investigated.
Prolactin
Human prolactin is similar in
structure to human growth hormone, and both are good lactogens. In women,
pro-lactin acts with other hormones on the mammary gland during pregnancy to
develop lactation and after birth to maintain it. Hyperprolactinemia causes
impotence in men and amenorrhea and infertility in women. Chronically el-evated
levels of circulating prolactin are associated with suppression of 17-
-estradiol and testosterone produc-tion in the ovaries and testes.
Prolactin serum levels
increase during pregnancy and breast-feeding, at least immediately after the
birth. In both men and women, prolactin increases after sleep starts, continues
to increase during the night, and in-creases markedly during stress. Prolactin
release is episodic during the day. More than 20 hormones and
neu-rotransmitters affect prolactin production, but the domi-nant physiological
control is primarily negative, mediated by dopamine from the hypothalamus.
Dopaminergic ago-nists inhibit prolactin release and antagonists, such as the
antipsychotic drugs, increase release.
There is no known therapeutic
use for prolactin, but serum levels are measured to diagnose
hyperprolactine-mia. The normal range of serum prolactin is 1 to 20 ng/mL.
Elevated prolactin levels ( 100 ng/mL) in the ab-sence of stimulatory factors,
such as antipsychotic drugs, are an indication of pituitary adenoma.
Approximately one-third of women who need treatment for infertility have high
serum prolactin levels. Galactorrhea, or inap-propriate lactation, is sometimes
associated with high prolactin levels. Hyperprolactinemia has been
tradition-ally treated by the dopaminergic agonist bromocriptine (Parodel). The doses, usually 5 mg/day,
are lower than those used to treat Parkinson’s disease, and therefore, the side
effects, nausea and postural hypotension, are less likely to cause problems.
More recently, however, the more potent, long-lasting dopaminergic agonist
cabergo-line (Dostinex) has been
found to be at least as effective and has a lower incidence of side effects.
Thyroid-Stimulating Hormone
TSH, or thyrotropin, is a
glycosylated protein of two subunits, and . TSH stimulates the thyroid gland to
produce thyroid hormones. Deficiencies are treated by giving thyroxine itself
rather than TSH, but TSH is available for diagnostic purposes to differentiate
be-tween pituitary and thyroid gland failure as causes of hypothyroidism .
Gonadotropins
Follicle-stimulating hormone
(FSH), luteinizing hor-mone (LH), and human chorionic gonadotropin (hCG) are
glycoproteins that are similar in structure to TSH. Glycosylation is not
identical among the different hor-mones, and the type of glycosylation
influences the half-life of the hormones. A sulfated N-acetylgalactosamine attached to LH but not FSH causes LH to be
more rap-idly metabolized; the half-life of LH is 30 minutes and that of FSH is
8 hours.
LH and FSH are pituitary
hormones secreted in pul-satile fashion approximately every 2 hours. In women
before menopause, this pattern is superimposed on much larger changes that
occur during the normal men-strual cycle. FSH is released in substantial
amounts dur-ing the follicular phase of the menstrual cycle and is re-quired
for proper development of ovarian follicles and for estrogen synthesis from
granulosa cells of the ovary. Most LH secretion occurs in an abrupt burst just
before ovulation. LH is required for progesterone synthesis in luteal cells and
androgen synthesis in thecal cells of the ovary. FSH stimulates spermatogenesis
and synthesis of androgen-binding protein in Sertoli cells of the testes. LH
stimulates testosterone production from Leydig cells. Production of LH and FSH
is controlled by gonadotropin-releasing hormone (GnRH) from the hy-pothalamus
and by feedback control from target organs through steroids and multiple forms
of a protein, in-hibin.
Injections of these hormones
are used to treat infertility in women and men. Traditional sources of
gonadotropins are from human urine. Human menopausal gonadotropins
(menotropins, Humegon, Pergonal) are isolated from urine of
postmenopausal women and contain both
FSH and LH. Purified prepa-rations of FSH from the same source are also
available (urofollitropin, Fertinex,
Fertinorm HP). During early pregnancy, trophoblasts of the placenta produce
hCG in large amounts. LH and hCG bind to the same go-nadal receptors, but hCG
is more stable and can be iso-lated from urine of pregnant women, so hGH
prepara-tions (Pregnyl, Profasi) are
used to mimic the burst of LH secretion before ovulation. Recombinant
prepara-tions of FSH are also available (follitropin, Gonal F, Follistim).
Gonadotropins are used to
treat infertility in women with potentially functional ovaries who have not
responded to other treatments. The therapy is de-signed to simulate the normal
menstrual cycle as far as is practical. A common protocol is daily injections
of menotropins for 9 to 12 days, until estradiol levels are equal to that in a
normal woman, followed by a single dose of hCG to induce ovulation. Two
problems with this treatment are risks of ovarian hyperstimulation and of
multiple births. Ovarian hyperstimulation is characterized by sudden ovarian
enlargement associ-ated with an increase in vascular permeability and rapid
accumulation of fluid in peritoneal, pleural, and pericardial cavities. To
prevent such occurrences, ovar-ian development is monitored during treatment by
ul-trasound techniques and by measurements of serum levels of estradiol.
Purified FSH is used to
prepare follicles for in vitro fertilization because LH activity in menotropins
may cause premature ovulation. Purified FSH is also used to treat infertility
in women with polycystic ovarian dis-ease; in this disease LH and androgen
production may already be elevated.
Gonadotropins are used to
induce spermatogenesis in hypogonadotropic hypogonadal men; a lengthy treat-ment
is required to obtain mature sperm. For several weeks hCG is injected to
increase testosterone levels, followed by injections of menotropins for several
months. Prepubertal cryptorchidism can be treated by injections of hCG for up
to several months.
Adrenocorticotropic Hormone
Adrenocorticotropic hormone
(ACTH), or corti-cotropin, a peptide of 39 amino acids, is first synthe-sized
as a larger precursor from which ACTH is de-rived by proteolytic cleavage. ACTH
stimulates production of glucocorticoids from the adrenal cortex . Release of
ACTH depends on diurnal rhythms with serum levels highest in the early morning.
Secretion of this peptide also increases under stress. It is easier and less
expensive to treat patients having adrenocortical insufficiency with
glucocorticoid re-placement therapy than it is to use ACTH. Therefore, use of
ACTH (Acthar) is restricted to
diagnosis; a shorter 24–amino acid analogue (Cosyntropin) is also used. Intravenous administration of ACTH
should re-sult in peak plasma levels of glucocorticoids within 30 to 60 minutes
if the adrenal gland is functional. Prolonged administration of ACTH in a
repository form, however, may be necessary to stimulate steroid production,
because ACTH has long-term trophic ef-fects on adrenal cells in addition to the
rapid stimula-tion of steroid production. If the cause of steroid defi-ciency
is at the level of the pituitary gland, ACTH should eventually stimulate
steroid production.
Related Topics