Chapter: Modern Pharmacology with Clinical Applications: Hypothalamic and Pituitary Gland Hormones
Hypothalamic Regulatory Hormones
HYPOTHALAMIC
REGULATORY HORMONES
Five peptides isolated from
the hypothalamus regulate release of one or more pituitary hormones. In
addition, dopamine released from the hypothalamus inhibits pro-lactin
production.
Somatostatin
Somatostatin (or somatotropin
release–inhibiting fac-tor [SRIF]) occurs primarily as a 14–amino acid
pep-tide, although a 28–amino acid form also exists. As with the other
hypothalamic peptides, it is formed by prote-olytic cleavage of a larger
precursor. Somatostatin, orig-inally isolated from the hypothalamus, is also in
many other locations, including the cerebral cortex, brain-stem, spinal cord,
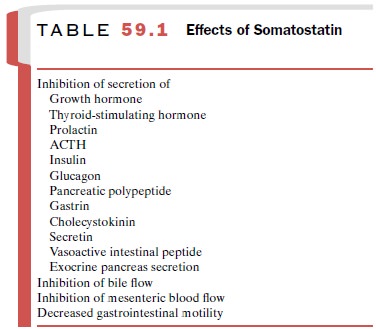
gut, urinary system, and skin. Somatostatin inhibits the secretion of many
substances in addition to growth hormone (Table 59.1).
Somatostatin has a very brief
half-life in serum and is not useful clinically. An 8–amino acid analogue with
2 D-amino acids substituted for
the naturally occurring L-amino acids is more stable, and monthly injections of a depot form of this analogue
(octreotide, Sandostatin LAR) have several uses. Long-acting
octreotide is used to treat
acromegaly, as described earlier. It is also used to counteract unpleasant
effects caused by overproduc-tion of secreted bioactive substances produced by
neu-roendocrine tumors, including hyperinsulinemia from insulinomas and
secretions from carcinoid tumors that cause severe diarrhea. Octreotide may
also control se-vere diarrhea associated with AIDS that has not re-sponded to
other treatments.
Transient side effects, gastrointestinal discomfort and decreased glucose tolerance, usually last only a few weeks after initiation of therapy. The most significant side effect associated with prolonged use of octreotide is formation of gallstones resulting from reduced bile flow.
Thyrotropin-Releasing Hormone
Thyrotropin-releasing hormone,
or protirelin, consists of three amino acids. TRH (Relefact TRH) is used for tests to distinguish primary from
secondary hypothy-roidism .
Gonadotropin-Releasing Hormone
GnRH (gonadorelin,
luteinizing hormone–releasing hormone) is a decapeptide that stimulates
production of LH and FSH. It is released in bursts from the hypo-thalamus at
regular intervals, about every 2 hours, al-though in women the interval may
lengthen in the luteal end of the menstrual cycle. The pituitary gland responds
to these regular pulses by producing LH and FSH. The pattern of LH and FSH in
cycling women, including the large burst of LH release before ovulation, can be
stim-ulated by regular administration of GnRH pulses. The large burst of LH
from the pituitary gland appears to be induced by feedback through estradiol
and other prod-ucts of the gonads that change the response of the pitu-itary
gland to the GnRH pulses rather than by large changes in the amounts of GnRH
secreted. The stimu-latory response to GnRH depends on pulsatile
adminis-tration and the timing of the pulses. Continual adminis-tration of GnRH
does not have the same effects as pulsatile administration; although production
of LH and FSH is stimulated initially, it is suppressed within a few days. Part
of this desensitization to GnRH is caused by a decrease in the number of
pituitary receptors for GnRH; additional postreceptor mechanisms are also
important in this complete suppression.
GnRH itself has a short
half-life, 7 minutes, if given intravenously. Structural variations of the
decapeptide have resulted in more stable analogues with higher affinity for the
GnRH receptor; a common modification is to substitute a D-amino acid for the sixth
amino acid, glycine, in GnRH.
Gonadotropin Stimulation
When stimulation of
gonadotropin production is needed, the pituitary gland is usually capable of
re-sponding to appropriately administered GnRH, even in cases of
hypogonadotropic hypogonadism, when LH and FSH levels are always low.
Therefore, GnRH ther-apy can be substituted for gonadotropin therapy by
ad-ministering GnRH (Lutrepulse)
pulses intravenously via an indwelling pump. GnRH itself is used, since the
short half-life is important to prevent accumulation be-tween pulses. The
advantage of this procedure com-pared with intramuscular injections of
gonadotropins
for treating infertility is
that normal levels of LH and FSH should be maintained because of feedback from
the gonads. This should reduce the risk of ovarian hy-perstimulation and
multiple births, since the procedure should not result in inappropriately high
levels of go-nadotropins (Table 59.2).
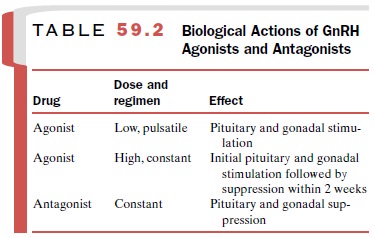
Gonadotropin Suppression
Stable potent derivatives of
GnRH include leuprolide (Lupron) and
goserelin (Zoladex). Because these
ago-nists are long acting, they suppress gonadotropin pro-duction after an
initial stimulation. In some uses, the ini-tial stimulation of gonadotropin is
undesirable; a newer GnRH antagonist, ganirelix (Antagon) inhibits go-nadotropin production without the stimulation
and may ultimately replace the long-acting agonists. These com-pounds are
formulated so they can be injected monthly or even less frequently.
In men, androgens stimulate
growth of prostatic cancer; therefore, a reduction in androgen actions is used
for palliative treatment . Estrogen use increases mortality in men primarily as
a result of cardiovascular complications, and castration is not pop-ular.
Therefore, treatment with GnRH analogues to suppress gonadotropin release is
favored. When long-acting agonists are given, signs and symptoms of prosta-tic
cancer may increase shortly after initiation of ther-apy because of the initial
stimulation of the pituitary gland. These analogues are also used to suppress
pu-berty in young children with central precocious puberty.
In women, GnRH agonists are sometimes given along with FSH when stimulating follicles in fertility treatments; this addition prevents premature ovulation caused by the release of pituitary LH. Uterine leiomy-omas and endometriosis regress when gonadotropin se-cretion is decreased. GnRH analogues relieve these conditions, but the relief usually lasts only as long as the analogue is administered, and the condition generally returns within a few months after therapy ceases.
The main side effects are a result of estradiol deprivation and include hot flashes
(sudden intense surface temper-ature elevation and sweating), dry skin and
vagina; long-term use may decrease bone density. The addition of estrogen and
progesterone can reduce the adverse ef-fects while maintaining gonadotropin
suppression. However, there is a continuing need to address the re-cent cancer
risk cautions issued for short-term versus long-term use of
estrogen–progesterone combinations as hormonal replacement therapy.
Corticotropin-Releasing Hormone
Corticotropin-releasing
hormone consists of 41 amino acids; it stimulates ACTH release. It is used for
investi-gational purposes.
Related Topics