Chapter: Essential Anesthesia From Science to Practice : Applied physiology and pharmacology : Anesthesia and the cardiovascular system
Anesthesia in the patient with cardiovascular disease
Anesthesia in the patient with
cardiovascular disease
Hypertension
When we
do not know the etiology, we hide behind the technical term “essential.” Thus,
we call “essential” the hypertension afflicting some 95% of patients. The
pathophysiology of essential hypertension is probably multi-factorial including
renal, vascular, cardiac, and neurohumoral factors – and reflex control
problems thrown in for good measure.
Chronic hypertension leads to left ventricular hypertrophy with consequent stiffening of the ventricle. A “stiffer,” less compliant ventricle will exhibit a large rise in intraventricular pressure during diastole. This increased diastolic pressure (wall tension) both increases myocardial oxygen demand and limits coronary perfusion. All organ perfusion depends on the upstream and downstream pres-sures. Thus, for the coronary perfusion pressure (CorPP):
CorPP = DBP − RAP or LVEDP
where
DBP = diastolic blood pressure (because the majority
of coronary perfu-sion occurs during diastole), RAP = right atrial pressure (where the coronaries empty, measured as
central venous pressure, CVP), and LVEDP = left ventricular end-diastolic pressure. With a stiff left
ventricle, LVEDP may exceed RAP and limit coronary perfusion, particularly in
the subendocardium. This combination leads to an increased risk of myocardial
ischemia (oxygen supply < demand). In addi-tion to its deleterious
effects on the heart, chronic hypertension leads to aortic, cerebral, and
peripheral vascular disease, as well as strokes and renal dysfunction.
In all
patients, particularly those we are going to anesthetize, we worry about
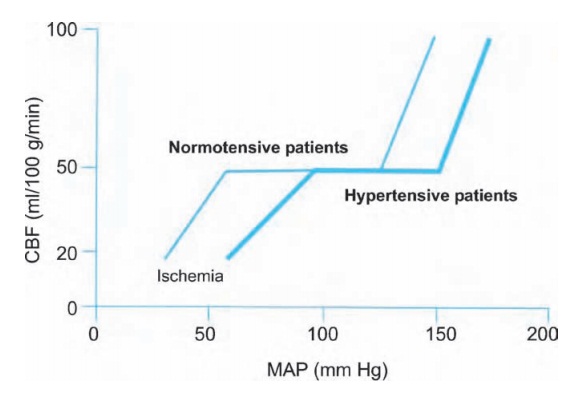
cerebral perfusion. The cerebral vasculature autoregulates to maintain a stable
blood flow over a range of mean arterial pressures. Chronic hypertension causes
a rightward shift of this cerebral autoregulation curve (see Fig. 9.5). An unfortunate side effect of this shift is
intolerance of low blood pressure. That is, a normoten-sive patient can
maintain cerebral blood flow down to a MAP of 50 mmHg; with chronic
hypertension, such a MAP might result in decreased cerebral perfusion and
possibly ischemia (decreased CNS function or even a stroke). While a con-scious
patient might complain of dizziness and perhaps become confused, under general
anesthesia we find it difficult to assess the adequacy of cerebral perfusion.
Thus, we
apply a general, albeit conservative, rule of thumb: maintain a patient’s blood
pressure within 20% of their baseline pressure.
The
anesthetic management of hypertension includes the following:
i.
Pre-operative control of blood pressure. We have data showing that
grossly hypertensive patients do poorly peri-operatively; we have no data that
would enable us to pinpoint the optimum of controlled hypertension.
ii.
Continuation of anti-hypertensive medication in the peri-operative
period, with the possible exception of ACE inhibitors, which have been linked
to refractory hypotension intra-operatively.
iii.
Intra-operative control of blood pressure swings. Hypertensive
patients are often volume depleted from chronic vasoconstriction or because
they take diuretics. Most anesthetics are vasodilators, and blood pressure can
fall pre-cipitously. Furthermore, the presence of anti-hypertensive drugs and
some anesthetics may interfere with the normal reflex response to hypotension.
As already mentioned, hypotension presents a particular risk to hyperten-sive
patients because they require increased diastolic pressure to maintain coronary
perfusion and may have impaired cerebral autoregulation.
Ischemic heart disease
Patients
with ischemic heart disease face significant risks when undergoing anes-thesia
and surgical procedures. In our pre-operative assessment, we must weigh
measures to protect them from peri-operative ischemia (see Pre-operative
evalu-ation). Diagnosing ischemia by electrocardiography (ST-segment
depression) can be difficult if a bundle branch block pattern obscures ST-segment
changes. Trans-esophageal echocardiography (TEE), which is minimally invasive
(but unpleasant if awake), can be quite helpful as it reveals wall motion
abnormalities, an early sign of ventricular dysfunction from coronary
insufficiency. TEE requires a skilled observer and expensive equipment (see
Monitoring).
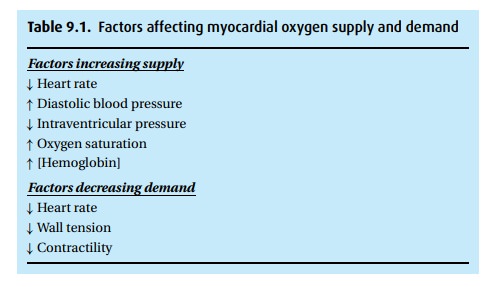
If we
suspect ischemia, remember physiology: ischemia means oxygen supply does not
meet demand. By looking at the factors affecting supply and demand, we can try
to improve conditions (Table 9.1).
First,
consider supply. Because the coronary
arteries are perfused during dias-tole, we want to maximize diastolic time
(lower heart rate) and coronary perfusion pressure (see above). For each amount
of blood that gets through, we want it to contain as much oxygen as possible
(see oxygen content equation in Anesthesia and the lung). Surprisingly, the
optimal hematocrit is actually only 30 mg/dL; at higher concentrations,
fluidity of blood decreases.
Now for demand. Cardiac contraction is “expensive” in an oxygen consumption sense, and the more contractions, the more “expense.” Thus, tachycardia has a dramatic impact on the supply : demand ratio, increasing oxygen consumption while at the same time reducing its supply. For this reason, heart rate reduction is a primary target during ischemic episodes. In addition, myocardial oxygen demand increases with increasing wall tension and, more importantly, contractility.
As of
this writing, peri-operative beta-receptor blockade receives much atten-tion.
It may have the potential to reduce cardiac deaths and complications (see
Pharmacology).
Pacemaker/AICD
More and
more patients are presenting with these life-saving devices (ACID, automatic
internal cardiac defibrillator) in place for heart rhythm disturbances (see
Pre-operative evaluation: Pacemaker/AICD for advance evaluation).
Intra-operatively, there are general rules for surgery in these patients:
(i) Enlist the help of cardiology colleagues to
check on the pacer function fol-lowing the operation.
(ii) Have a magnet on hand. This nifty low-tech
device reverts most pacemakers into a back-up paced-only mode at a rate
dependent on the manufacturer, program, and remaining battery life.
(iii) Avoid electromagnetic interference. We do not
want the pacemaker to become part of the electrocautery circuit, so we consider
the route between the surgical site and the electrocautery grounding pad and
make sure it does not cross the pacemaker or its leads.
(iv) With rate-responsive pacemakers, we might avoid
agents that fool the device into thinking its owner is running a marathon
(succinylcholine-induced fasciculations, shivering).
(v) Disable AICDs to prevent inappropriate shock
when the device is confused by electrocautery.
(vi) Keep electrolytes normal, particularly K+ and Mg2+ .
Following
conclusion of the operation, the pacer may require reprogramming, and the AICD
should be reactivated.
Congestive heart failure
Congestive
heart failure (CHF) describes a heart that is not pumping well. Ordin-arily, the
heart dilates to accept blood at a low filling pressure, then propels it
forward forcefully with each contraction. CHF can result from pathology at
several points in the pump’s function:
(i)Poor ventricular
compliance A non-compliant ventricle, as may occur with ischemia or
hypertrophy, will exhibit substantial increases in pressure at even “normal”
filling volumes. This will impede ventricular filling and increase the pressure
in the venous system. In approximately one-third of CHF patients, this diastolic dysfunction predominates as
the mechanism for their disease.
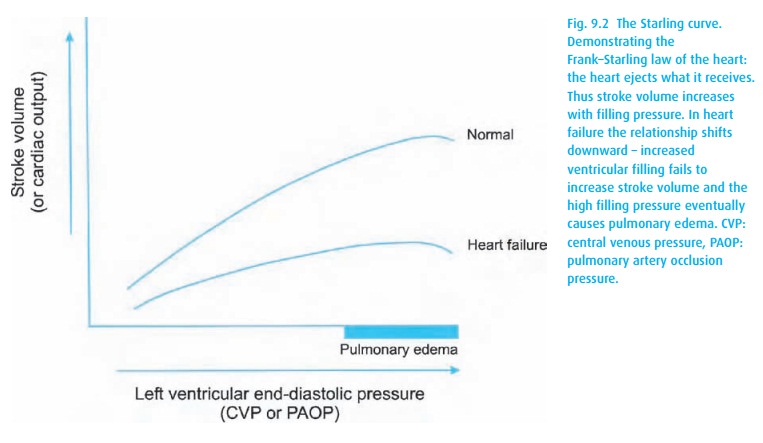
(ii)
The descending limb of Starling’s
curve Though a bit controversial, there may be a point at which further
increasing diastolic filling actually results in a decreasing stroke volume. Here, substantial increases in
ventricular pressure can result in pulmonary congestion and edema. A reduction
in preload can move the heart back to the more functional side of the curve
(see Fig. 9.2) and reduce the filling
pressure sufficient to alleviate pulmonary congestion.
(iii)
Contractility In Fig.9.2, the Starling curve of the CHF patient
resides lowerand runs flatter than normal, reflecting the high filling
pressures required to generate even a marginal stroke volume.
(iv)
Afterload Increased afterload is the most common cause of
hypertension.The increased force of contraction required to eject against this
afterload is deleterious to a failing heart.
The
importance of these influences suggests the current treatment regimen for the
most common cause of CHF, left ventricular systolic dysfunction, namely
inotropic support with digoxin, diuretics to decrease preload, and afterload
reduc-tion with an ACE inhibitor.
Related Topics