Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Genitourinary Surgery
Anesthesia for Lithotripsy
LITHOTRIPSY
The treatment of kidney stones has
evolved from primarily open surgical procedures to less invasive or entirely noninvasive
techniques. Cystoscopic procedures, including flexible ureteroscopy with stone
extraction, stent placement, and intracorpo-real lithotripsy (laser or
electrohydraulic), along with medical
expulsive therapy (MET), have become first-line therapy. Extracorporeal
shock wave litho-tripsy (ESWL) is also utilized, primarily for 4-mm to 2-cm
intrarenal stones, and percutaneous and lapa-roscopic nephrolithotomy for
larger or impacted stones. MET has become the treatment of choice among many
clinicians for acute episodes of uroli-thiasis: for stones up to 10 mm in
diameter, admin-istration of the α blockers tamsulosin (Flomax), doxazosin
(Cardura), or terazosin (Hytrin) or the calcium channel blocker nifedipine
(Procardia, Adalat) lessens the pain of acute urolithiasis and increases the
rate of stone expulsion over a period of several days to several weeks.
During ESWL, repetitive high-energy
shocks (sound waves) are generated and focused on the stone, causing it to
fragment as tensile and shear forces develop inside the stone and cavitation
occurs on its surface. Water or a conducting gel couples the generator to the
patient. Because tissue has the same acoustic density as water, the waves
travel through the body without damaging tissue. However, the change in
acoustic impedance at the tissue–stone interface creates shear and tear forces
on the stone. Subsequently, the stone is fragmented enough to allow its passage
in small pieces down the urinary tract. Ureteral stents are often placed
cystoscopi-cally prior to the procedure. Tissue destruction can occur if the
acoustic energy is inadvertently focused at air–tissue interfaces such as in
the lung and intes-tine. The inability to position the patient so that lung and
intestine are away from the sound wave focus is a contraindication to the
procedure. Other con-traindications include urinary obstruction below the
stone, untreated infection, a bleeding diathe-sis, and pregnancy. The presence
of a nearby aor-tic aneurysm or an orthopedic prosthetic device is considered a
relative contraindication. Ecchymosis, bruising, or blistering of the skin over
the treatment site is not uncommon. Rarely, a large perinephric hematoma can
develop and may be responsible for a postoperative decrease in
hematocrit.Electrohydraulic, electromagnetic, or piezo-electric shock wave
generators may be used for
ESWL. With older electrohydraulic units, the patient is placed
in a hydraulic chair and immersed in a heated water bath, which conducts the
shock waves to the patient. Modern lithotriptors gener-ate shock waves either
electromagnetically or from piezoelectric crystals. The generator is enclosed
in a water-filled casing and comes in contact with the patient via a conducting
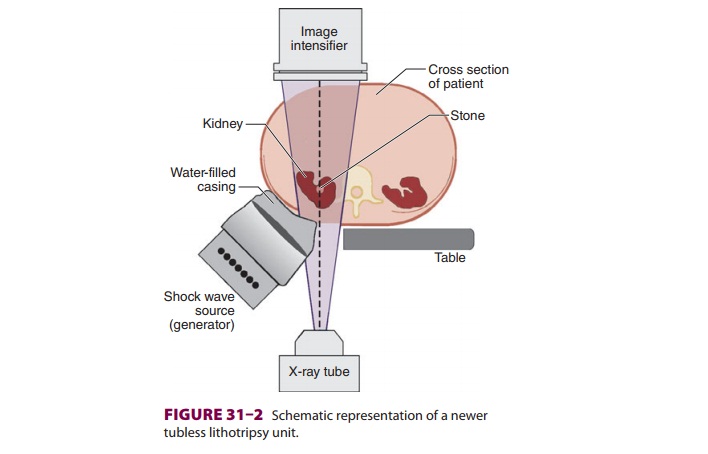
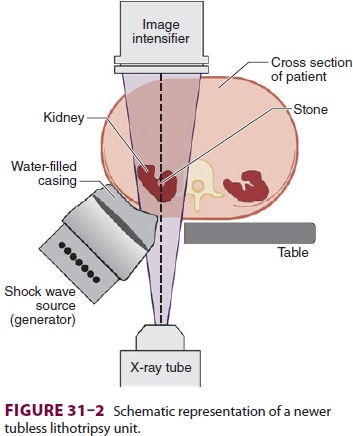
gel on a plastic mem-brane (Figure 31–2). Newer units allow
both fluo-roscopic and ultrasound localization. In the case of electromagnetic
machines, the vibration of a metallic plate in front of an electromagnet
pro-duces the shock waves. With piezoelectric models, the waves are the result
of changes in the external dimensions of ceramic crystals when electric
cur-rent is applied.
Preoperative Considerations
Patients with a history of cardiac
arrhythmias and those with a pacemaker or internal cardiac defibrillator (ICD)
may be at risk for developing arrhythmias induced by shock waves during ESWL.
Synchronization of the shock waves with the electro-cardiogram (ECG) R wave
decreases the incidence of arrhythmias during ESWL. The shock waves are usually
timed to be 20 ms after the R wave to correspond with the ventricular
refractory period. Studiessuggest that asynchronous delivery of shocks may be
safe in patients without heart disease. Shock waves can damage the internal
components of pacemaker and ICD devices. The manufacturer should be con-tacted
as to the best method for managing the device (eg, reprogramming or applying a
magnet).
Intraoperative Considerations
Anesthetic considerations for ureteroscopy, stone manipulation,
and laser lithotripsy are similar to those for cystoscopic procedures. ESWL
requires special considerations, particularly when older lith-otriptors requiring
the patient to be immersed in water are used.
A. Effects of Immersion During ESWL
Immersion into a heated water bath
(36–37°C) ini-tially results in vasodilation that can transiently lead to
hypotension. Arterial blood pressure, however, subsequently rises as venous
blood is redistributed centrally due to the hydrostatic pressure of water on
the legs and abdomen. Systemic vascular resistance (SVR) rises and cardiac
output often decreases. The sudden increase in intravascular volume and SVR can
precipitate congestive heart failure in patients with marginal cardiac reserve.
Moreover, the increase in intrathoracic blood volume reduces functional
residual capacity 30–60% and may pre-dispose some patients to hypoxemia.
B. Choice of Anesthesia
Pain during lithotripsy is from
dissipation of a small amount of energy as shock waves enter the body through
the skin. The pain is therefore localized to the skin and is proportionate to
the shock wave intensity. Older water bath lithotripsy units require 1000–2400
relatively high-intensity shock waves, which most patients cannot tolerate
without either regional or general anesthesia. In contrast, newer lithotripsy
units that are coupled directly to the skin utilize 2000–3000 lower-intensity
shock waves that usually require only light sedation.
C. Regional Anesthesia
Continuous epidural anesthesia is
commonly employed when ESWL utilizes older water bath lithotriptors. Regional
anesthesia with sedation greatly facilitates positioning and monitoring in this
situation, and supplemental oxygen by face mask or nasal cannula is also useful
in avoiding hypoxemia. A T6 sensory level ensures adequate anesthesia, as renal
innervation is derived from T10 to L2. Supplementation of the block with
epidural fentanyl (50–100 mcg) is often useful. When using the loss of
resistance technique for placement of the epidural catheter, saline should be
used instead of air during epidural catheter insertion; as air in the epidural
space can dissipate shock waves and may promote injury to neural tissue. Foam
tape should not be used to secure the epidural catheter as this type of tape
has been shown to dissipate the energy of the shock waves when it is in their
path. Spinal anesthesia can also be used satisfactorily but offers less control
over the sensory level and an uncertain duration of surgery; for this reason,
epidural anes-thesia is usually preferred.
A major disadvantage of regional anesthesia or sedation is the
inability to control diaphragmatic movement. Excessive diaphragmatic excursion
during spontaneous ventilation can move the stone in and out of the wave focus
and may prolong the procedure. This problem can be partially solved by asking
the patient to breathe in a more rapid but shallow respiratory pattern.
Bradycardia due to high sympathetic blockade also prolongs the pro-cedure when
shock waves are coupled to the ECG, and small doses of glycopyrrolate are often
admin-istered in this situation to accelerate the ESWL procedure.
D. General Anesthesia
General endotracheal anesthesia allows
control of diaphragmatic excursion during lithotripsy using older water bath
lithotriptors. The procedure is complicated by the inherent risks associated
with placing a supine anesthetized patient in a chair, ele-vating and then
lowering the chair into a water bath to shoulder depth, and then reversing the
sequence at the end. A light general anesthetic technique in conjunction with a
muscle relaxant is preferable. The muscle relaxant ensures patient immobility
and con-trol of diaphragmatic movement.
E. Monitored Anesthesia Care
Light intravenous sedation with midazolam and fentanyl is
usually adequate for modern low-energy lithotripsy. Deeper sedation with
low-dose propofol infusions with or without midazolam and opioid
supplementation may also be used.
F. Monitoring
Standard anesthesia monitoring must be
used for conscious or deep sedation, or for general anesthesia.
Even with
R-wave synchronized shocks, supraven-tricular
arrhythmias can occur. With
immersionlithotripsy, ECG pads should be attached securely with waterproof
dressing. Changes in functional residual capacity with immersion mandate
monitor-ing of oxygen saturation, particularly in patients at risk for
developing hypoxemia. The temperature of the bath and the patient should be
monitored to pre-vent hypothermia or hyperthermia.
G. Fluid Management
Intravenous fluid therapy is typically
generous. Following an initial intravenous fluid bolus, an addi-tional
1000–2000 mL of lactated Ringer’s injection is often given with a small dose of
furosemide to maintain brisk urinary flow and flush stone debris and blood
clots. Patients with poor cardiac reserve require more conservative fluid
therapy.
Related Topics