Chapter: Pharmaceutical Drug Analysis: Amperometric Methods
Amperometric Methods
AMPEROMETRIC METHODS
INTRODUCTION
An amperometric
method or amperometry is
concerned with the measurement of current under a constant applied voltage ;
and under such experimental parameters the concentration of the ‘analyte’ exclusively determines the
quantum and magnitude of the current. Hence, these measurements may be employed
effectively to record the alteration in concentration of an ion in question in
the course of a titration, and ultimately the end-point is established. This
specific process is commonly referred to as amperometric
method or amperometry.
In this particular case, the total current flowing shall
remain almost equal to the current carried by the ions that undergoes equal
electrolytic migration together with the current caused on account of the
diffusion of the ions. Thus, we have :
I = Id + Im
where I = Total current,
Id = Diffusion current, and
Im = Migration current.
An awkward situation arises when dealing with a dilute
solution where it has been observed that the depletion of the electrode layer
ultimately leads to an enhancement of the resistance of the solution and
thereby affecting subsequently an alteration in the Ohm’s Law potential drop (I
× R) in the cell. This ulti-mately gives rise to a doubtful observed potential
operative at the electrode. In order to overcome this serious anomaly, it is a
normal practice to add an excess of an indifferent electrolyte to the system,
such as : 0.1 M KCl, which renders the solution to remain stable at a low and
constant resistance, whereas the migration current (Im) of the species under
examination almost vanishes i.e., I =
Id.
The ion under investigation, whose rate of diffusion at
the electrode surface is governed by Fick’s
Law represented as under :
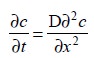
where, D = Diffusion coefficient,
C = Concentration,
t = Time, and
x = Distance from the electrode
surface.
Thus, the potential of the electrode is controlled and
monitored by the Nernst Equation as
shown below:
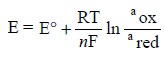
Salient Features of Amperometric Methods :
The various salient features of amperometric titrations are enumerated below :
(a) It is less
dependent upon the characteristics of the electrode,
(b) It is quite
independent of the nature and type of the supporting electrolyte,
(c) It does not
require a constant temperature in the course of a titration but it should not
necessarily be fixed accurately,
(d) The
substance under investigation may not essentially be reactive at the electrode
; whereas either a reactive reagent or a product is just sufficient for a
successful amperometric titration, and
(e) The amperometric method is
inherently more accurate and precise, and therefore, has an edge as compared to
the polarographic method.
Related Topics