Chapter: Obstetrics and Gynecology: Puberty
Abnormalities of Pubertal Development
ABNORMALITIES OF PUBERTAL DEVELOPMENT
The abnormalities of puberty
include precocious puberty, primary amenorrhea, delayed sexual maturation, and
in-complete sexual maturation.
The
presence of any of these disorders requires investigation of both the
hypothalamic–pituitary–gonadal axis as well as the reproductive outflow tract.
The initial evaluation should
begin with measurement of pi-tuitary gonadotropin (follicle-stimulating hormone
[FSH] and luteinizing hormone [LH]) levels, which helps distin-guish a
hypothalamic–pituitary etiology from a gonadal etiology.
Precocious Puberty
Precocious puberty is the onset
of secondary sexual charac-teristics prior to the age 6 in black girls and age
7 in white
Precocious puberty is caused by either GnRH-dependentor GnRH-independent
sex hormone production (Box 34.1).GnRH-dependent, or true (central)
precocious puberty, develops secondary to early activation of the hypothalamic–
pituitary–gonadal axis. The most common causes are idiopathic; other causes
include infection, inflammation, or injury of the central nervous system. In
idiopathic pre-cocious puberty, the arcuate nucleus in the hypothalamus is
prematurely activated. This causes early sexual maturation with early
reproductive capability. The elevated estrogen levels affect the skeleton,
resulting in short stature in adult-hood secondary to premature closure of the
epiphyseal plates. These individuals are at risk for sexual abuse and have
psychosocial problems related to their early sexual develop-ment. Occasionally,
GnRH-dependent precocious puberty results from neoplasms of the
hypothalamic–pituitary stalk. In this situation, although sexual development
begins early, the rate of sexual development is slower than usual. Transient
inflammatory conditions of the hypothalamus may also result in GnRH-dependent
precocious puberty; however, sexual development may begin and end abruptly.
Laboratory studies show either an appropriate rise in go-nadotropins or a
steady gonadotropin level in the prepuber-tal range.
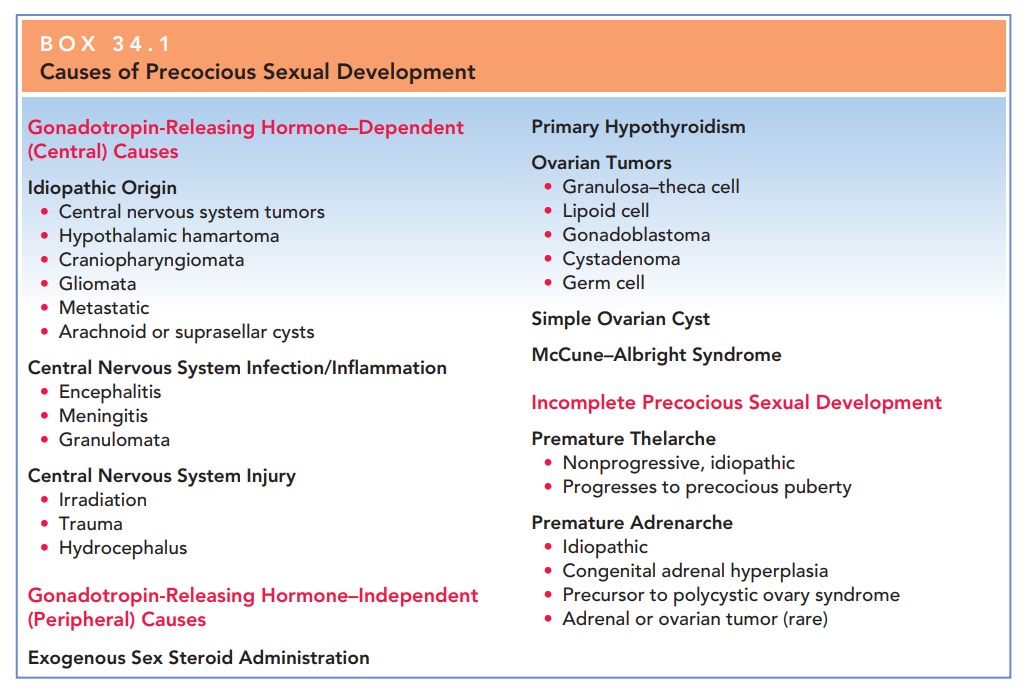
GnRH-independent
sex hormone production, or precocious pseudopuberty (peripheral), results from
sex hormone production (androgens or estrogens) independent of
hypothalamic–pituitary stimulation. This
condition can be caused by ovarian cystsor tumors, McCune–Albright syndrome,
adrenal tumors, or iatrogenic causes. Some tumors, such as granulosa cell
tumors, teratoma, or dysgerminomata, directly secrete androgen. Physical
examination usually reveals a palpable pelvic mass and leads to further
evaluation/imaging studies.
McCune–Albright
syndrome (polyostotic fibrous dysplasia) is characterized by multiple bone
fractures, café-au-lait spots, and precocious puberty. Premature
menarche can be the first sign
The syndrome is
thought to result from a defect in cellular regulation with a mutation in the
alpha subunit of the G protein that stimulates cAMP formation, which causes
affected tissues to function autonomously. This mutation causes the ovary to
produce estrogen with-out the need for FSH, resulting in sexual precocity.
Adrenal
causes of precocious puberty include adrenal tumors or enzyme-secreting
defects, such as congenital adrenal hyperpla-sia (CAH). Tumors
are very rare and must secrete estrogento cause early sexual maturation. The
most common form of CAH, 21-hydroxylase deficiency, presents at birth with the
finding of ambiguous genitalia. However, the nonclassical form, previously
known as late-onset CAH, tends to present at adolescence. In this disorder, the
adrenal glands are unable to produce adequate amounts of cortisol as a result
of a partial block in the conversion of 17-hydroxyprogesterone to
deoxycortisol. Deficiency of the 21-hydroxylase enzyme leads to a shunting away
from aldosterone and cortisol production in cholesterol biosyn-thesis toward
the production of androgens (testosterone and estradiol), which results in
precocious adrenarche. Apathognomonic
finding for 21-hydroxylase deficiency is an elevated 17-hydroxyprogesterone
level. Plasma renin is also measured todetermine the amount of
mineralocorticoid deficiency. Medical therapy is instituted as early as
possible and is aimed at steroid/mineralocorticoid replacement, depending on
the severity of the deficiency. In the nonclassical form of CAH, patients
present with premature adrenarche, anovulation, and hyperandrogenism, appearing
somewhat like patients with polycystic ovarian syndrome.
Iatrogenic
causes such as drug ingestion must be considered in all children who present
with precocious puberty.
These children may exhibit
increased pigmentation of the nipples and areola of the breast secondary to
ingestion of oral contraceptives, anabolic steroids, and hair or facial creams.
The main
goals of treatment of precocious puberty are to ar-rest and diminish sexual
maturation until a normal pubertal age, as well as to maximize adult height. Therapy
for GnRH-independent precocious puberty involves administration of a GnRH
agonist. Results occur rapidly and continue during the first year of treatment.
Treatment for GnRH-independent precocious puberty attempts to suppress go-nadal
steroidogenesis.
Delayed Puberty
There is wide variation in normal
pubertal development. However, puberty is
considered delayed when secondary sex characteristics have not appeared by age
13, there is no evidence of menarche by age 15 to 16, or when menses have not
began 5 years after the onset of thelarche. These findings shouldprompt the
physician to initiate a workup to determine the cause of the delay. The most
common causes of delayed puberty are shown in Box 34.2.
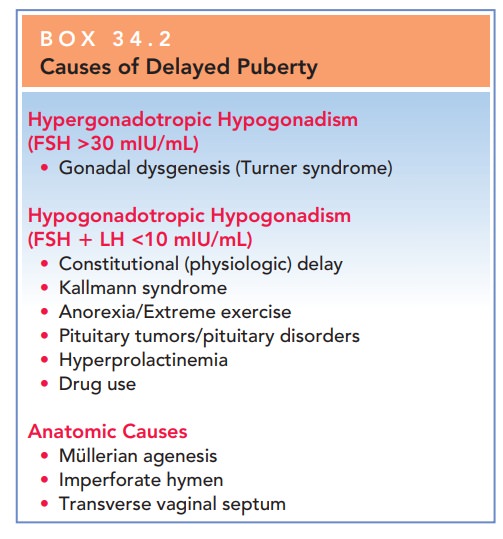
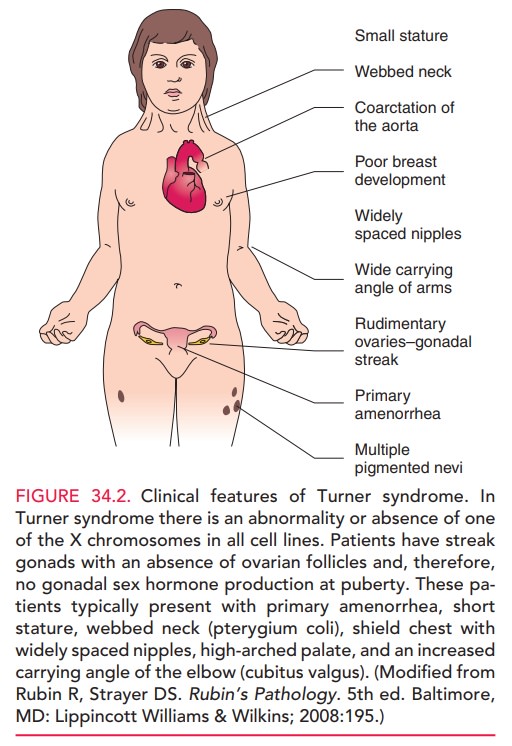
HYPERGONADOTROPIC HYPOGONADISM
The most
common cause of delayed puberty with an elevated FSH is gonadal dysgenesis, or
Turner syndrome. In this condition,there is an
abnormality in or absence of one of the X chro-mosomes in all cell lines.
Patients have streak gonads, with an absence of ovarian follicles; therefore,
gonadal sex hor-mone production does not occur at puberty. These pa-tients
typically have primary amenorrhea, short stature, webbed neck (pterygium coli),
shield chest with widely spaced nipples, high arched palate, and an increased
carry-ing angle of the elbow (cubitus valgus) [see Fig. 34.2].
Estrogen
administration should be initiated at the normal time of initiation of puberty,
and growth hormone should be initiated very early (often prior to estrogen
therapy) and ag-gressively to normalize adult height.
Estrogen is necessary to
stimulate breast development, genital tract maturation, and the beginning of
menstrua-tion. Low-dose estrogen is used to initiate secondary sexual
maturation, and the dose is increased once breast budding and menarche occur.
If an excessive amount of
Box 34.2
Causes of Delayed Puberty
Hypergonadotropic Hypogonadism (FSH >30 mIU/mL)
·
Gonadal
dysgenesis (Turner syndrome)
Hypogonadotropic Hypogonadism (FSH LH <10 mIU/mL)
·
Constitutional
(physiologic) delay
·
Kallmann
syndrome
·
Anorexia/Extreme
exercise
·
Pituitary
tumors/pituitary disorders
·
Hyperprolactinemia
·
Drug
use
Anatomic Causes
·
Müllerian
agenesis
·
Imperforate
hymen
· Transverse vaginal septum
estrogen is administered
initially, epiphyseal closure may begin, and long bone growth is truncated and
adult height compromised. A delay in estrogen administration can lead to the
development of osteoporosis in the teenage years. Progestins should not be
given until the patient has reached Tanner stage IV, because premature
progestin therapy may prevent the breast from developing completely, thus
result-ing in an abnormal contour (a more tubular breast).
HYPOGONADOTROPIC HYPOGONADISM
The arcuate nucleus of the
hypothalamus secretes GnRH in cyclic bursts (or a pulsatile fashion), which
stimulates release of gonadotropins from the anterior pituitary gland. Dysfunction
of the arcuate nucleus disrupts the short hormonal loop between the
hypothalamus and pi-tuitary. As a result, FSH and LH secretion does not occur.
Consequently, the ovaries are not stimulated to secrete estradiol, and
secondary sexual maturation is delayed. Themost
common cause of this type of delayed puberty is constitutional (physiologic)
delay. Other causes include Kallmann syndrome;anorexia, exercise, or
stress; pituitary tumors/pituitary dis-orders; hyperprolactinemia; and drug
use.
Constitutional delay of puberty
represents approxi-mately 20% of all cases of delayed puberty. It is thought to bea normal variant of the
development process and trends can be seen within families. Children with
constitutional delay usuallyhave not only delay of secondary sexual maturation,
but also short stature with an appropriate delay of bone maturation.
In the
Kallmann syndrome, the olfactory tracts are hypoplas-tic, and the arcuate
nucleus does not secrete GnRH. Youngwomen with Kallmann syndrome
have little or no sense of smell and do not have breast development. This
condition can be diagnosed on initial physical examination by chal-lenging the
olfactory function with known odors such as coffee or rubbing alcohol. Once the
condition is recog-nized and treated, the prognosis for successful secondary
sexual maturation and reproduction is excellent. Secondary sexual maturation
can be stimulated by the administration of exogenous hormones or by the
administration of pul-satile GnRH. Patients typically can have normal reproduc-tive
function. Ovulation is induced by the administration of exogenous gonadotropin,
and progesterone is given in the luteal phase to allow implantation of the
embryo.
Other
causes of hypothalamic amenorrhea include weight loss, strenuous exercise (such
as ballet dancing or long-distance running), anorexia nervosa, or bulimia. These
conditions allresult in suppressed gonadotropin levels with low estrogen
levels. The correction of the underlying abnormality (such as weight gain in
patients with weight loss) restores normal gonadotropin levels, stimulating
ovarian steroidogenesis and the resumption of pubertal development.
Craniopharyngioma
is the most common tumor associated with delayed puberty. This
tumor develops in the pituitarystalk with suprasellar extension from nests of
epithelium de-rived from the Rathke pouch. The radiologic hallmark is the
appearance of a (supra)sellar calcified cyst. Calcifications are present in
approximately 70% of craniopharyngiomas.
ANATOMIC CAUSES
During fetal life, müllerian
ducts develop and fuse in the female fetus to form the upper reproductive tract
(i.e., the fallopian tubes, uterus, and upper vagina). The lower and midportion
of the vagina develop from the canalization of the genital plate.
Müllerian
agenesis, or Mayer-Rokitansky–Küster–Hauser syndrome, is the most common cause
of primary amenorrhea in women with normal breast development. In this
syndrome,there is congenital absence of the vagina and usually an ab-sence of
the uterus and fallopian tubes. Ovarian function is normal, because the ovaries
are not derived from müller-ian structures; therefore, all the secondary sexual
charac-teristics of puberty occur at the appropriate time. Physical examination
leads to the diagnosis of müllerian agenesis. Renal anomalies (e.g.,
reduplication of the ureters, horse-shoe kidney, or unilateral renal agenesis)
occur in 40% to 50% of cases. Skeletal anomalies such as scoliosis occur in 10%
to 15% of cases. Mayer-Rokitansky–Küster–Hauser syndrome is generally sporadic in
expression, although oc-casional occurrences in families can be seen.
There are several therapeutic
approaches to this con-dition. Nonsurgical approaches should be tried first,
using dilators and pressure on the dimple between the urethra and the rectum,
twice a day. This tissue is quite pliable and, with increasing dilator size, a
normal-length vagina can be achieved. An artificial vagina may be created by
repetitive pressure by vaginal dilators on the perineum or by surgi-cal
construction followed by a split-thickness skin graft. After creation of a
vagina, these women are able to have sexual intercourse. With the advances in
assisted repro-ductive technologies, including in vitro fertilization (IVF) and
use of a surrogate mother (gestational carrier), it is possible for a woman
with this condition to have a genetic child by using her oocytes.
The
simplest genital tract anomaly is imperforate hymen. In this
condition, the genital plate canalization is incom-plete, and the hymen is,
therefore, closed. Menarche occurs at the appropriate time, but because there
is obstruction to the passage of menstrual blood, it is not apparent. This
condition presents with pain in the area of the uterus and a bulging,
bluish-appearing vaginal introi-tus. Hymenotomy is the definitive therapy. This
condi-tion may be confused with a transverse vaginal septum. Transverse vaginal
septa can occur along the vagina at any level and result in obstruction to
outflow of menses. A vaginal septum can be resected and primarily repaired via procedure
called a Z-vaginoplasty. Prolonged obstruc-tion to menstruation can be
associated with an increased incidence of endometriosis.
Related Topics