Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Antibacterial and Antiviral Agents
β-Lactam Antimicrobics - Antimicrobics That Act on Cell Wall Synthesis
β-Lactam Antimicrobics
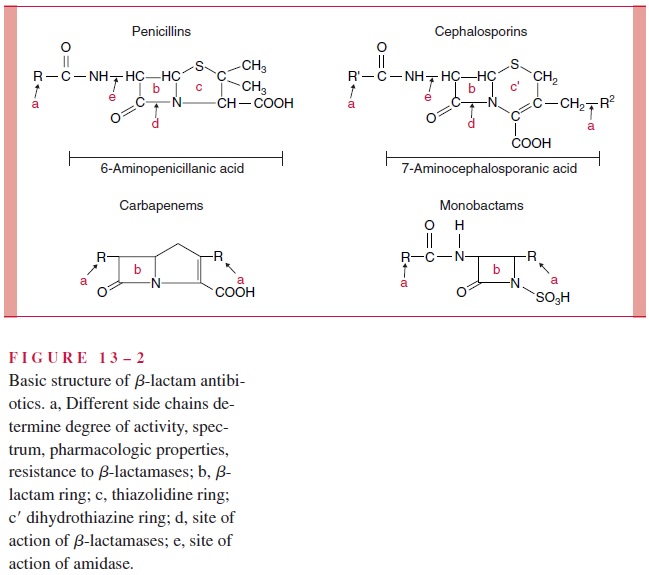
The β-lactam antimicrobics comprise the penicillins, cephalosporins, carbapenems, and monobactams. Their name derives from the presence of a β-lactam ring in their structure; this ring is essential for antibacterial activity. Penicillin, the first member of this class, was derived from molds of the genus Penicillium, and later natural β-lactams were derived from both molds and bacteria of the genusStreptomyces. Today it is possible to synthesize β-lactams, but most are derived from semisynthetic processes involving the chemical modification of the products of fermentation.
The β-lactam antimicrobics interfere with the transpeptidation reactions that seal the peptide crosslinks between glycan chains. They do so by interference with the action of the transpeptidase enzymes which carry out this cross-linking. These targets of all the β-lactams are commonly called penicillin-binding proteins (PBPs), reflecting the stereo-chemical nature of their interference, which was first described in experiments with peni-cillin. Several distinct PBPs occur in any one strain, are usually species specific, and vary in the avidity of their binding to different β-lactam antimicrobics.
The β-lactams are classified by chemical structure (Fig 13 – 2). They may have one β-lactam ring (monobactams), or a β-lactam ring fused to a five-member penem ring (penicillins, carbapenems), or a six-member cephem ring (cephalosporins). Within these major groups, differences in the side chain(s) attached to the single or double ring can have a significant effect on the pharmacologic properties and spectrum of any β-lactam. The pharmacologic properties include resistance to gastric acid, which allows oral administra-tion, and their pattern of distribution into body compartments (eg, blood, cerebrospinal fluid, joints). The features that alter the spectrum include permeability into the bacterial cell, affinity for PBPs, and vulnerability to the various bacterial mechanisms of resistance.
β-lactam antimicrobics are usually highly bactericidal, but only to growing bacteria syn-thesizing new cell walls. Killing involves attenuation and disruption of the developing pepti-doglycan “corset,” liberation or activation of autolytic enzymes that further disrupt weakened areas of the wall, and finally osmotic lysis from passage of water through the cytoplasmic membrane to the hypertonic interior of the cell. As might be anticipated, cell wall – deficient organisms, such as Mycoplasma, are not susceptible to β-lactam antimicrobics.
Penicillins Penicillins differ primarily in their spectrum of activity against Gram-negative bacteria and resistance to staphylococcal penicillinase. This penicillinase is one of a family of bacterial enzymes called β-lactamases that inactivate β-lactam antimicro-bics .
Penicillin G is active primarily against Gram-positive organisms, Gram-negative cocci, and some spirochetes, including the spirochete of syphilis. They have little action against most Gram-negative bacilli, because the outer membrane prevents passage of these antibiotics to their sites of action on cell wall synthesis. Penicillin G is the least toxic and least expensive of all the penicillins. Its modification as penicillin V confers acid stability, so it can be given orally.
The penicillinase-resistant penicillins (methicillin, nafcillin, oxacillin) also have nar-row spectra, but are active against penicillinase-producing S. aureus. The broader spectrum penicillins owe their expanded activity to the ability to traverse the outer membrane of some Gram-negative bacteria. Some, such as ampicillin, have excellent activity against a range of Gram-negative pathogens but not P. aeruginosa, an important opportunistic pathogen.
Others, such as carbenicillin and ticarcillin, are active against Pseudomonas when given in high dosage but are less active than ampicillin against some other Gram-negative organisms. The penicillins with a Gram-negative spectrum are slightly less active than penicillin G against Gram-positive organisms and are inactivated by staphylococcal penicillinase.
Cephalosporins The structure of the cephalosporins confers resistance to hydrolysis bystaphylococcal penicillinase and to the β-lactamases of groups of Gram-negative bacilli, which vary with each cephalosporin. The cephalosporins are classified by generation — first, second, third, or fourth. The “generation” term relates to historical breakthroughs in expanding their spectrum through modification of the side chains. In general, a cephalosporin of a higher generation has a wider spectrum, in some instances, more quan-titative activity (lower minimum inhibitory concentration; MIC) against Gram-negative bacteria. As the Gram-negative spectrum increases, these agents typically lose some of their potency (higher MIC) against Gram-positive bacteria.
The first-generation cephalosporins cefazolin and cephalexin have a spectrum of ac-tivity against Gram-positive organisms that resembles that of the penicillinase-resistant penicillins, and in addition, they are active against some of the Enterobacteriaceae (see Table 13 – 1). These agents continue to have therapeutic value because of their high activ-ity against Gram-positive organisms and because a broader spectrum may be unnecessary.
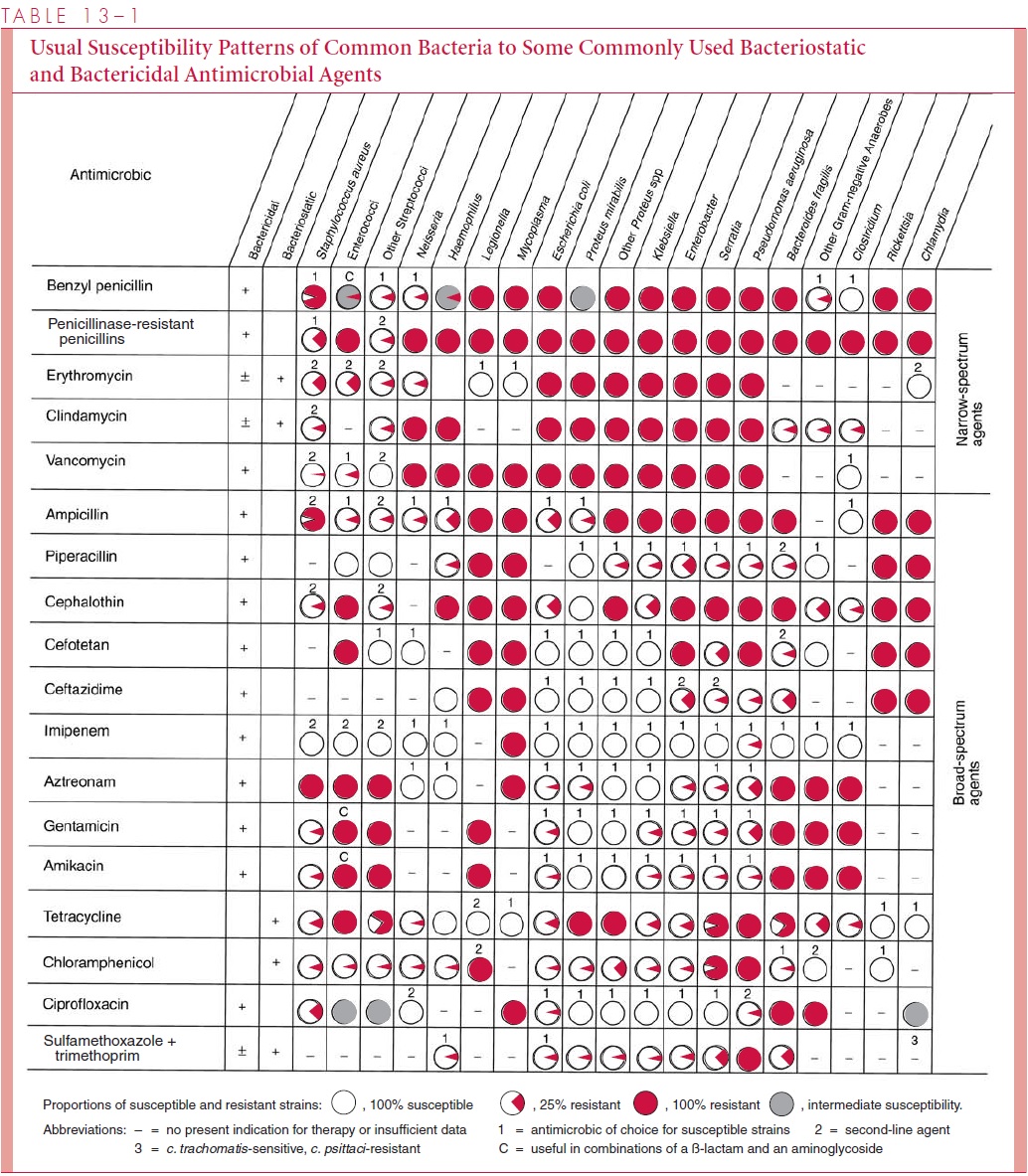
Second-generation cephalosporins cefoxitin and cefaclor are resistant to β-lactamases of some Gram-negative organisms that inactivate first-generation compounds. Of particular importance is their expanded activity against Enterobacteriaceae species and against anaer-obes such as Bacteroides fragilis.
Third-generation cephalosporins, such as ceftriaxone, cefotaxime, and ceftazidime, have an even wider spectrum; they are active against Gram-negative organisms, often at MICs that are 10- to 100-fold lower than first-generation compounds. Of these three agents, only ceftazidime is consistently active against P. aeruginosa. The potency, broad spectrum, and low toxicity of the third-generation cephalosporins have made them the preferred agents in life-threatening infections in which the causative organism has not yet been isolated. Selection depends on the clinical circumstances. For example, ceftriaxone or cefotaxime is preferred for childhood meningitis because it has the highest activity against the three major causes, Neisseria meningitidis, Streptococcus pneumoniae, andHaemophilus influenzae. For a febrile bone marrow transplant patient, ceftazidime mightbe chosen because of the prospect of P. aeruginosa involvement.
Fourth-generation cephalosporins have enhanced ability to cross the outer membrane of Gram-negative bacteria as well as resistance to many Gram-negative β-lactamases. Compounds such as cefepime have activity against an even wider spectrum of Enterobac-teriaceae as well as P. aeruginosa. These cephalosporins retain the high affinity of third-generation drugs and activity againstNeisseria and H. influenzae.
Carbapenems The carbapenems imipenem and meropenem have the broadest spectrum of all β-lactam antibiotics. This fact appears to be due to the combination of easy penetration of Gram-negative and Gram-positive bacterial cells and high level of resis-tance to β-lactamases. Both agents are active against streptococci, more active than cephalosporins against staphylococci, and highly active against both β-lactamase-positive and -negative strains of gonococci and H. influenzae. In addition, they are as active as third-generation cephalosporins against Gram-negative rods, and effective against obli-gate anaerobes. Imipenem is rapidly hydrolyzed by a renal tubular dehydropeptidase-1; therefore, it is administered together with an inhibitor of this enzyme (cilastatin), which greatly improves its urine levels and other pharmacokinetic characteristics. Meropenem is not significantly degraded by dehydropeptidase-1 and does not require coadministration of cilastatin.
Monobactams Aztreonam,the first monobactam licensed in the United States, has aspectrum limited to aerobic and facultatively anaerobic Gram-negative bacteria, includ-ing Enterobacteriaceae, P. aeruginosa, Haemophilus, and Neisseria. Monobactams have poor affinity for the PBPs of Gram-positive organisms and anaerobes and thus little activity against them, but they are highly resistant to hydrolysis by β-lactamases of Gram-negative bacilli. Anaerobic superinfections and major distortions of the bowel flora are less common with aztreonam therapy than with other broad-spectrum β-lactam antimicrobics, presumably because aztreonam does not produce a general suppression of gut anaerobes.
β-lactamase Inhibitors A number of β-lactams with little or no antimicrobic activityare capable of binding irreversibly to β-lactamase enzymes and, in the process, rendering them inactive. Three such compounds, clavulanic acid, sulbactam, and tazobactam, are referred to as suicide inhibitors, because they must first be hydrolyzed by a β-lactamase before becoming effective inactivators of the enzyme. They are highly effective against staphylococcal penicillinases and broad-spectrum β-lactamases; however, their ability to inhibit cephalosporinases is significantly less. Combinations of one of these inhibitors with an appropriate β-lactam antimicrobic protects the therapeutic agent from destruction by many β-lactamases and significantly enhances its spectrum. Four such combinations are now available in the United States: amoxicillin/clavulanate, ticarcillin/clavulanate, ampi-cillin/sulbactam, and piperacillin/tazobactam. Bacteria that produce chromosomally encoded inducible cephalosporinases are not susceptible to these combinations. Whether these combi-nations offer therapeutic or economic advantages compared with the β-lactamase – stable antibiotics now available remains to be determined.
Clinical Use The β-lactam antibiotics are usually the drugs of choice for infections bysusceptible organisms because of their low toxicity and bactericidal action. They have also proved of great value in the prophylaxis of many infections. They are excreted by the kidney and achieve very high urinary levels. Penicillins reach the cerebrospinal fluid when the meninges are inflamed and are effective in the treatment of meningitis, but first- and second-generation cephalosporins are not. In contrast, the third-generation cephalosporins penetrate much better and have become the agents of choice in the treatment of undiagnosed meningitis and meningitis caused by most Gram-negative organisms.
Related Topics