Physical and Chemical properties | Lipids - Triacylglycerols or Triglycerides | 11th Biochemistry : Chapter 6 : Lipids
Chapter: 11th Biochemistry : Chapter 6 : Lipids
Triacylglycerols or Triglycerides
Triacylglycerols or Triglycerides
Triacylglycerols
are simple lipids synthesized by esterification of glycerol with three
molecules fatty acids. It is the storage form of fat, stored in adipose tissue.
They are hydrolyzed in the gut by lipases to free fatty acids and
monoglycerides.
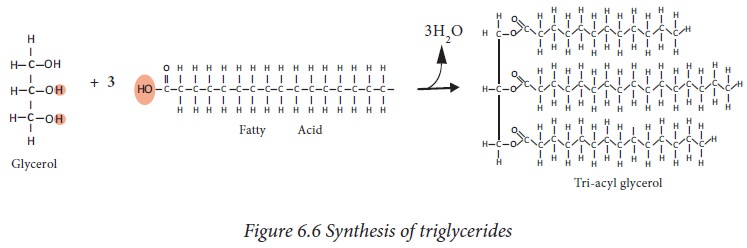
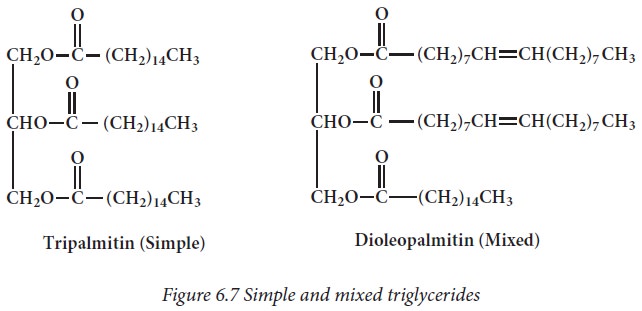
If the
three hydroxyl groups of glycerol molecule are esterified with the same type of
fatty acid then the lipid is called simple triacyl glycerol eg: tripalmitin. If
esterification is with different fatty acids, it is called as mixed glycerides
eg: dioleopalmitin.
Properties
Physical properties
·
They are non-polar, hydrophobic, insoluble in water and soluble in
organic solvents.
·
Specific gravity is less than water. Therefore fats and oil float on
water.
·
They serve as solvent for other fats. Example: Fat soluble vitamins A,
D, E and K
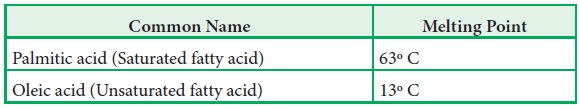
·
The saturated fatty acids have higher melting points than unsaturated
fatty acids of corresponding length.
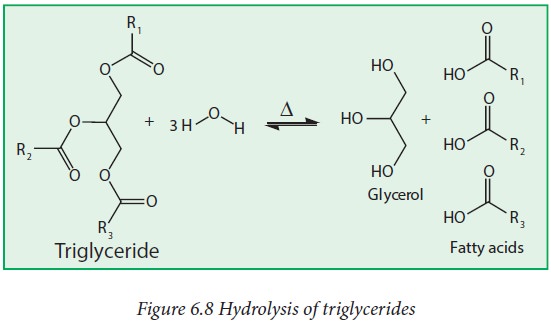
Chemical properties
i. Hydrolysis:
Triglycerides (fats) can be hydrolyzed to produce glycerol and
fatty acids in the presence of acid and heat or with a suitable lipase enzyme
under biological conditions.
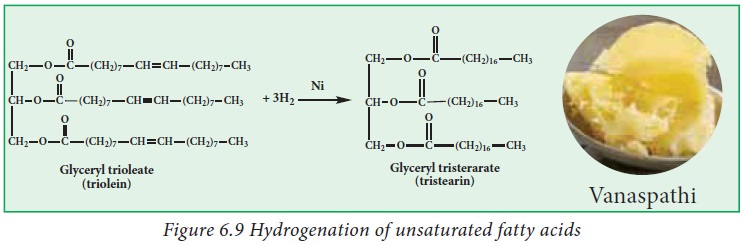
ii. Hydrogenation:
Hydrogenation
is a process of adding hydrogen atoms to unsaturated fats until they become
saturated. Hydrogenation of fat is a process used in industries, food
manufacturers, to synthesize modified plant fats called hydrogenated fats that
share similar texture and taste characteristics with saturated animal fats.
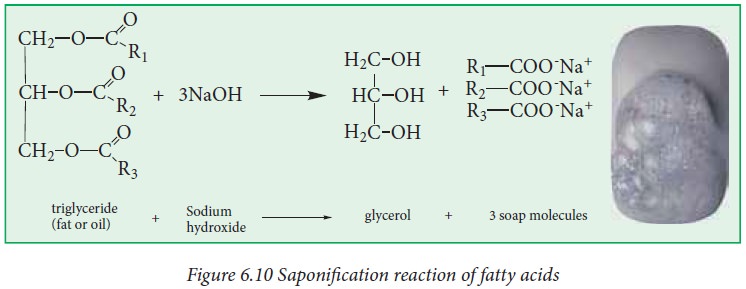
iii. Saponification:
The
process of hydrolysis of fat by aqueous alkali (NaOH or KOH) to yield glycerol
and the salt of fatty acid (soap) is called saponification or alkaline
hydrolysis of esters. Soaps are sodium or potassium salts of long chain fatty
acids.
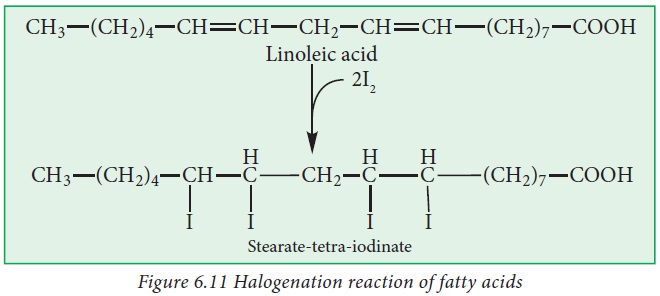
iv. Halogenation:
Unsaturated
fatty acids have the ability to bind halogens like Cl2, Br2
and I2 to their double bonds. It is a very important property which
determines the degree of unsaturation of the fat or oil that determines its
biological value.
v. Rancidity:
Rancidity
is a term generally used to denote unpleasant odours and flavours in foods
resulting from deterioration in the fat or oil portion of a food. The
triacylglycerols in fats with low molecular mass carboxylic acids undergo
oxidation very quikly when exposed to air, moisture and light or hydrolysed in
the presence of bacterial lipases.
Related Topics