Chapter: 10th Science : Chapter 3 : Thermal Physics
Temperature
TEMPERATURE
Temperature is defined as the degree of hotness of a body. The temperature is higher for a hotter body than for a colder body. It is also be defined as the property which determines whether a body is in equilibrium or not with the surroundings. (or average kinetic enegy of the molecules). Further, temperature is the property, which determines the direction of flow of heat. It is a scalar quantity. The SI unit of temperature is kelvin (K). There are other commonly used units of temperature such as degree celsius (°C) and degree fahrenheit (°F).
1. Absolute scale (kelvin scale) of temperature
The temperature measured in relation to absolute zero using the kelvin scale is known as absolute temperature. It is also known as thethermodynamic temperature. Each unit of the thermodynamic scale of temperature is defined as the fraction of 1/273.16th part of the thermodynamic temperature of the triple point of water. A temperature difference of 1°C is equal to that of 1K. Zero Kelvin is the absolute scale of tempeture of the body.
The relation between the different types of scale of temperature:
Celsius and Kelvin: K=C+273,
Fahrenheit and Kelvin: [K] = (F + 460) × 5/9
0K = –273°C.
2. Thermal equilibrium
Two or more physical systems or bodies are said to be in thermal equilibrium if there is no net flow of thermal energy between the systems. Heat energy always flows from one body to the other due to a temperature difference between them. Thus, you can define thermal equilibrium in another way. If two bodies are said to be in thermal equilibrium, then, they will be at the same temperature. What will happen if two bodies at different temperatures are brought in contact with one other? There will be a transfer of heat energy from the hot body to the cold body until a thermal equilibrium is established between them. This is depicted in Figure 3.1.
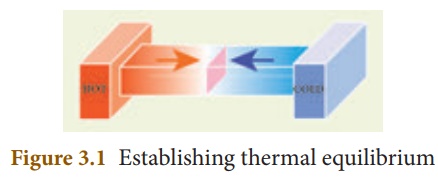
When a cold body is placed in contact with a hot body, some thermal energy is transferred from the hot body to the cold body. As a result, there is some rise in the temperature of the cold body and decrease in the temperature of the hot body. This process will continue until these two bodies attain the same temperature.
Related Topics