Chapter: Basic Concept of Biotechnology : Plant Molecular Farming: A Promising Stratergy in Biotechnology
Subcellular Targeting of proteins in plants
Subcellular Targeting of proteins in plants
Organelle-specific protein targeting, protein sequestration in, or targeting to a specific cell compartments has also been readily recognized as a key factor determining the overall stability and yield of recombinant proteins in plants (Wandelt et al., 1992; Schouten et al., 1996; Gomord et al., 1997). Targeting signals can be used to intentionally retain recombinant proteins within distinct compartments of the cell to protect them from proteolytic degradation, preserve their integrity and to increase their accumulation levels (Seon et al., 2002). Several subcellular compartments have been considered as possible destinations for recombinant proteins in plant cells, endoplasmic reticulum, chloroplast and different subcompartments of the cell secretory pathway (Ma et al., 2003; Daniell, 2006; Goulet and Michaud, 2006). Recombinant products and their localization sites in the plant are depicted in figure 1.
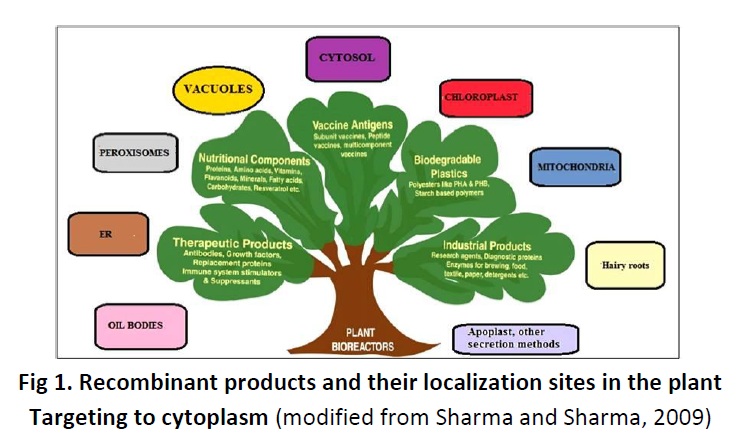
The absence of a targeting signal in the transgene sequence prevents migration of the recombinant protein out of the cytosol following mRNA translation. Recombinant proteins retained in the cytosol are usually detected at very low levels, accumulation rates below 0.1% of TSP (Conrad and Fiedler, 1998). Several factors may explain the limited suitability of the cytosol as a destination for recombinant proteins: The negative redox potential of the cytosolic milieuis unfavorable to the correct folding of proteins with disulphide bonds (Goulet and Michaud, 2006); The important co and post-translational modification processes, such as glycosylation and the effective housekeeping activity of the ubiquitin–proteasome proteolytic pathway which may have a positive impact on the folding and assembly, structural stability of several nascent and mature proteins was absent in this compartment (Faye et al., 2005, Vierstra, 1996, 2003).
Cytosolic targeting of the tomato mosaic virus antibody ‘rAb29’ in tobacco leaf cells, for instance, resulted in very weak accumulation rates, whereas the same transgene including a signal peptide for
extracellular secretion produced easily detectable amounts of this protein (Schillberg et al., 1999). Similarly, retaining human growth hormone in the cytosol of Nicotiana benthamiana leaf cells led to protein levels of about 0.01% of TSP, in contrast with concentrations reaching 10% of TSP for the same protein targeted to the apoplast (Gils et al., 2005). Cytosolic accumulation of the human growth factor, forinstance, had toxic effects in leaves ofNicotiana benthamiana whereas, in contrast, no negative effects were observed for the same protein targeted to the chloroplast (Gils et al., 2005). Likewise, the bovine protease inhibitor (aprotinin) was accumulated at high specific levels when retained in the endoplasmic reticulum (ER) of potato leaf cells (Badri, 2006). Although some recombinant proteins remain stable in the cytosol (e.g. Michaud et al., 1998; De Jaeger et al., 1999), alternative destinations, such as the chloroplast, the ER or the apoplast, appear to be more appropriate for most proteins.
Targeting to the endoplasmic reticulum
Proteins bearing a signal peptide for cellular secretion first enter the endoplasmic reticulum (ER) via the ER protein translocation channel (Galili et al., 1998), and then migrate through this compartment to the golgi apparatus until reaching the extracellular medium (default pathway) or the vacuole, if a vacuolar sorting signal is found in the primary sequence. Recombinant proteins entering the ER may also be retained in this compartment by simple apposition of the tetrapeptide ER retention signal (K /H) DEL (Michaud et al., 1998). Plants also accumulate many proteins which do not have a C-terminal retention signal in the ER e.g. prolamins. ER retention signal from γ-zein, a prolamin of maize, has been characterized which is more efficient than KDEL signal (Mainieri et al., 2004). Using γ-zein signal, the foreign protein accumulated up to 3.5% of total extractable protein, whereas, it was only0.5% when KDEL sequence was used. Also, as the protein bodies are insoluble (Mainieri et al., 2004), they can be easily purified by centrifugation and it further makes the production of recombinant protein economical. Further, oil bodies originate from ER and store plant seed oils. They are surrounded by a phospholipid monolayer and their membrane is rich in the protein oleosin. A foreign protein could be fused to oleosin with a protease cleavage site in between. This strategy has been used to produce the thrombin inhibitor hirudin from seeds of transgenic Brassica napus(Parmenter et al., 1995).
Endoplasmic reticulum is considered as a suitable destination for several proteins because of the presence of low abundance of proteolytic enzymes and the presence of molecular chaperones in the ER, together with an oxidizing status favouring disulphide bond formation, helping the protein for proper folding (Nuttall et al., 2002; Faye et al., 2005). Similar tendencies have been observed for several other proteins of medical or industrial interest. For example human interleukin-4 (Ma et al., 2005), the SARS coronavirus S protein antigen (Pogrebnyak et al., 2005), the synthetic silk-like protein DR1B (Yang etal., 2005) and a recombinant phytase from Aspergillus niger(Peng et al.,2006). Despite these promising developments, the ER cannot be considered as a suitable destination for all proteins. To be stable or active, a number of clinically useful proteins require late post-translational modifications, such as the formation of complex glycans, the addition of a lipid moiety or the proteolytic removal of a propeptide sequence, which may occur downstream of the ER along the secretory pathway, notably in the Golgi, vacuole or apoplast (Gomord and Faye, 2004; Faye et al., 2005). Proteins may exhibit an altered integrity or structural heterogeneity in the ER, as a result of unintended proteolytic processing by ER-resident proteases (Faye et al., 2005). For instance, the bovine plasma protein aprotinin expressed in leaves of transgenic potatoshowed structural heterogeneity when accumulated in the Endoplasmic reticulum, presumably as a result of the sequential removal of specific amino acids at the N- and C- termini by endogenous peptidases (Badri etal., 2005).
Targeting to the peroxisome and nucleus
The recombinant proteins may be sent to these organelles by the inclusion of an appropriate targeting peptide (or localization signal) in the transgene sequence (Daniell et al., 2002; Hyunjong et al., 2006). For instance a fungal xylanase showed high accumulation levels in Arabidopsis leaf tissues sent to peroxisomes using the tripeptidetargeting motif SKL (serine-lysine-leucine) grafted at the C-terminus (Hyunjong et al., 2006).
Targeting to the chloroplast
Efficient procedures have also been devised to insert transgenes in the chloroplast genome, and then to regenerate transplastomic plant lines accumulating high levels of recombinant protein directly in the chloroplast (Daniell et al., 2001b; Maliga, 2002). It eliminates the position effect and results in uniform expression of transgene (Daniell etal., 2001a). Human therapeutic protein, interferon gamma was fused toHis-tagged GUS and 6% of total soluble protein was expressed in tobacco chloroplasts (Leelavathi and Reddy, 2003). In the tobacco chloroplasts Cry1Ia5 protein accumulated up to 3% of TSP in the leaf tissue (Reddy etal., 2002). De Cosa et al., (2001) reported exceptionally highaccumulation of Bt Cry2Aa2 proteins (up to 46% of TSP) when the transgene was engineered in chloroplasts. Recently, plastoglobules, the sub-chloroplastic compartments, have, been targeted for recombinant protein accumulation (Vidi et al., 2007). Like the oil bodies they are light in weight and contain only a few proteins (Ytterberg et al., 2006), which is advantageous for purification. Chlorogen, a biotechnological company,has adopted the chloroplast transformation as platform technology for the production of foreign proteins in plants. Several transplastomic plant lines have been engineered over the last 10 years for recombinant protein expression, providing very high yields for a number of useful proteins of prokaryotic and eukaryotic origin, including somatotropin, serum albumin, anthrax protective antigen, cholera toxin B subunit and tetanus toxin fragment C (Daniell et al., 2001, 2005; Tregoninget al., 2003) The chloroplast transformation vector contains two targeting sequences that flank the transgene and insert them through homologous recombination at a particular site.
Chloroplast transformation offers several advantages over nuclear transformation, including uniform transgene expression rates, multiple copies of the transgene in each cell, co-expression of multiple genes from the same construct, minimal gene silencing and minimal transgene escape in the environment owing to the maternal inheritance of chloroplast DNA in several species (Daniell et al., 2002). However, a number of endogenous proteases are present in the chloroplast, which can impair the overall stability and accumulation of recombinant proteins (Adam and Clarke, 2002). An interesting example has been provided for the rotavirus VP6 protein, which showed high accumulation rates in chloroplasts of young tobacco leaves, but negligible rates in older leaves (Birch-Machin et al., 2004). A similar decline in older tissues was observed for a fungal xylanase (Hyunjong et al., 2006) and for the insecticidal Bt toxin Cry2Aa2 (De Cosa et al., 2001). Another disadvantage of the chloroplast transgenic system is that plastids do not carry out glycosylation. It is therefore unlikely that chloroplasts could be used to synthesize human glycoproteins in cases in which the glycan-chain structure is crucial for protein activity.
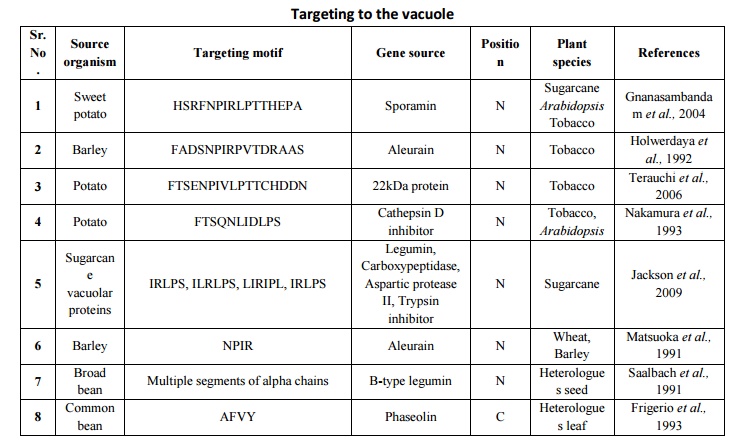
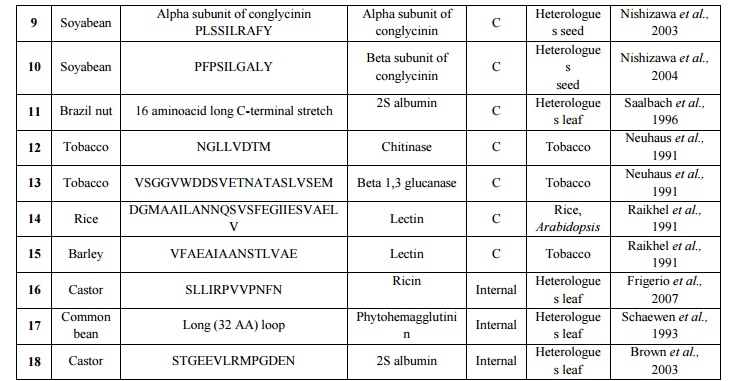
Table 4.List of various vacuolar targeting motifs used in plantmolecular farming Plant vacuolesnot only maintain the cell turgor but also store proteins and secondary metabolites. There are two distinct types of vacuole in plant cells: lytic (or vegetative) vacuoles, which has an acidic environment rich in hydrolytic enzymes; and protein storage vacuoles, which show a slightly acidic or neutral pH well adapted to protein storage (Robinson et al., 2005). Signal sequences that are responsible for targeting the protein to the vacuoles have been identified (Brown et al., 2003; Jolliffe et al., 2004; Matsuoka and Neuhaus, 1999) but no consensus protein sorting signal has been optimized so far. High accumulation levels have been reported for a numberof recombinant proteins targeted to the vacuole including, synthetic analogue of spider dragline silk protein DP1B in Arabidopsisthaliana(Yang et al., 2005), E. coli heat-labile enterotoxin B in tobacco(Streatfield et al., 2003), the toxic biotin-binding proteins avidin and streptavidin in tobacco (Murray et al., 2002), Asgergillus niger phytase in maize (Arcalis et al., 2004) and a thermo stable glucanase of bacterial origin in barley (Horward et al., 2004).In contrast to protein storing vacuoles, vacuoles of vegetative tissues like leaves have higher hydrolytic activity and recombinant protein targeted would degrade. Therefore, mechanisms or the signals required to store recombinant proteins in the vacuoles need further exploration. A correct in situ localization of recombinant proteins bearing a sorting sequence for vacuolar targeting should be undertaken on a systematic basis, considering the species- or tissue-dependent functionality of some sorting signals (Vitale and Hinz, 2005). A very well example of this phenomenon is silk-like protein DP1B expressed in Arabidopsis (Yang et al., 2005), shows a tissue specific expression of protein reaching 8% of TSP in seed storage vacuoles, and no detectable levels of the same protein could be observed in leaf cell vacuoles. In a similar manner, targeting DP1B to the apoplast providedgood yields in leaves, but poor yields in seeds (Yang et al., 2005), again stressing the need for an empirical case-by-case assessment of different tissue and cellular destinations for each protein expressed.A list of various vacuolar targeting motifs used in plant molecular farming is given in table 4. Theimpact of sub cellular targeting on recombinant protein yield in transgenic plant systems was given in table 5.
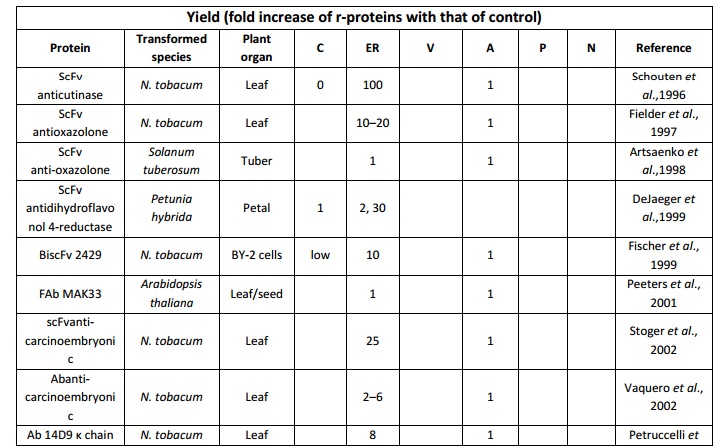
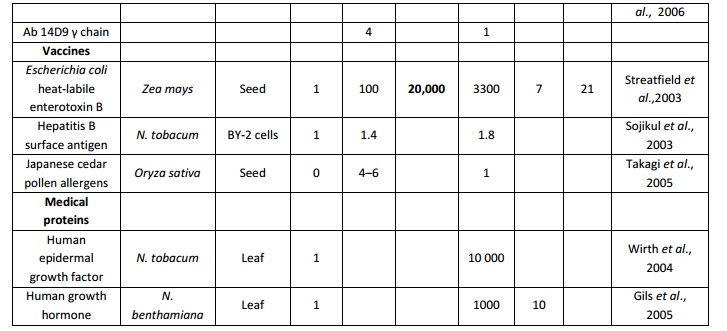
Table 5. Impact of subcellular targeting of recombinant protein yield in transgenic plant systems (modified from Benchabaneet al.,2008) C-cytoplasm; ER-Endoplasmic reticulum; V-Vacuole; N-Nucleus; A-Apoplast and P- Peroxisomes
Related Topics