Chapter: Medical Surgical Nursing: Assessment of Musculoskeletal Function
Structure and Function of the Skeletal System
Anatomic
and Physiologic Overview
The health and proper
functioning of the musculoskeletal system is interdependent with that of the
other body systems. The bony structure provides protection for vital organs,
including the brain, heart, and lungs. The bony skeleton provides a sturdy
framework to support body structures. The bone matrix stores calcium,
phos-phorus, magnesium, and fluoride. More than 98% of the total-body calcium
is present in bone. In addition, the red bone marrow located within bone
cavities produces red and white blood cells in a process called hematopoiesis.
Joints hold the bones together and allow the body to move. The muscles attached
to the skeleton con-tract, moving the bones and producing heat, which helps to
main-tain body temperature.
STRUCTURE AND FUNCTION OF THE SKELETAL SYSTEM
There are 206 bones in the human body, divided into four
categories:
·
Long bones (eg, femur)
·
Short bones (eg, metacarpals)
·
Flat bones (eg, sternum)
·
Irregular bones (eg,
vertebrae)
The shape and
construction of a specific bone are determined by its function and the forces
exerted on it. Bones are constructed of cancellous
(trabecular) or cortical (compact)
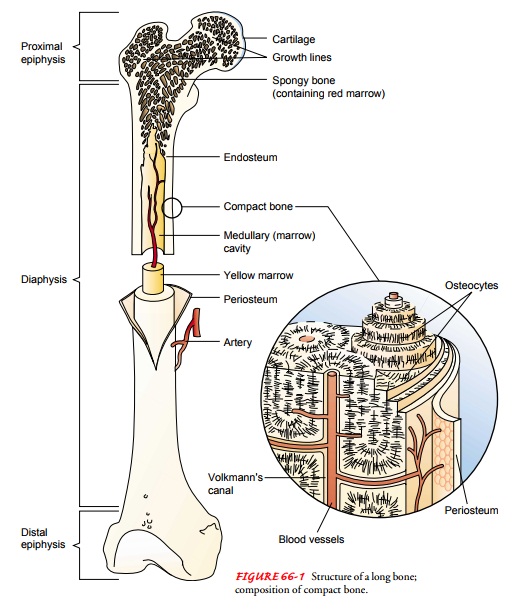
bone tissue. Long bones are shaped like rods or shafts with rounded ends (Fig.
66-1). The shaft, known as the diaphysis,
is primarily cortical bone. The ends of the long bones, called epiphyses, are primarily can-cellous
bone. The epiphyseal plate separates the epiphyses from the diaphysis and is
the center for longitudinal growth in chil-dren. In the adult, it is calcified.
The ends of long bones are cov-ered at the joints by articular cartilage, which is a tough, elastic,
avascular tissue. Long bones are designed for weight bearing and movement.
Short bones consist of cancellous bone covered by a layer of compact bone. Flat
bones are important sites for hemato-poiesis and frequently provide vital organ
protection. They are made of cancellous bone layered between compact bone.
Irregu-lar bones have unique shapes related to their functions. Generally,
irregular bone structure is similar to that of flat bones.
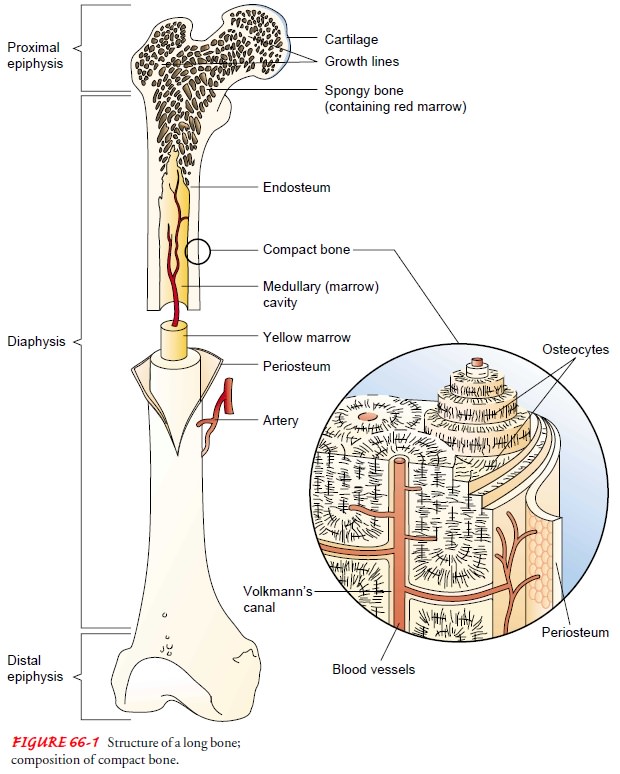
Bone is composed of cells, protein matrix, and mineral de-posits. The cells are of three basic types—osteoblasts, osteocytes, and osteoclasts. Osteoblasts function in bone formation by se-creting bone matrix. The matrix, which consists of collagen and ground substances (glycoproteins and proteoglycans), provides a framework in which inorganic mineral salts are deposited. Osteo-cytes are mature bone cells involved in bone-maintenance func-tions; they are located in lacunae (bone matrix units). Osteoclasts, located in shallow Howship’s lacunae (small pits in bones), are multinuclear cells involved in destroying, resorbing, and remold-ing bone. The microscopic functioning unit of mature cortical bone is the osteon (Haversian system). The center of the osteon, the Haversian canal, contains a capillary. Around the capillary are circles of mineralized bone matrix called lamellae. Within the lamellae are lacunae containing osteocytes. These are nourished through tiny structures, canaliculi (canals), that communicate with adjacent blood vessels within the Haversian system (see Fig. 66-1).
Lacunae in cancellous
bone are layered in an irregular lattice network (trabeculae). Red bone marrow fills the lattice network. Capillaries
nourish the osteocytes located in the lacunae.
Covering the bone is a
dense, fibrous membrane known as the periosteum.
The periosteum nourishes bone and allows for itsgrowth; it also provides for
the attachment of tendons and liga-ments. The periosteum contains nerves, blood
vessels, and lym-phatics. The layer closest to the bone contains osteoblasts,
which are bone-forming cells.
The endosteum
is a thin, vascular membrane that covers the marrow cavity of long bones and
the spaces in cancellous bone. Osteoclasts, which dissolve bone to maintain the
marrow cavity, are located near the endosteum in Howship’s lacunae.
Bone marrow is a
vascular tissue located in the medullary (shaft) cavity of long bones and in
flat bones. Red bone marrow, located mainly in the sternum, ilium, vertebrae,
and ribs in adults, is responsible for producing red and white blood cells. In
adults, the long bone is filled with fatty, yellow marrow.
Bone tissue is well
vascularized. Cancellous bone receives a rich blood supply through metaphyseal
and epiphyseal vessels. Periosteal vessels carry blood to compact bone through
minute Volkmann’s canals. In addition, nutrient arteries penetrate the
periosteum and enter the medullary cavity through foramina (small openings).
Nu-trient arteries supply blood to the marrow and bone. The venous system may
accompany arteries or may exit independently.
Bone Formation (Osteogenesis)
Bone begins to form long
before birth. Ossification is the
process by which the bone matrix (collagen fibers and ground substance) is
formed and hardening minerals (eg, calcium salts) are deposited on the collagen
fibers. The collagen fibers give tensile strength to the bone, and the calcium
provides compressional strength.
There are two basic
processes of ossification: endochondral and intramembranous. Most bones in the
body are formed by en-dochondral ossification, in which a cartilage-like tissue
(osteoid) is formed, resorbed, and
replaced by bone. Intramembranous os-sification occurs when bone develops
within membrane, as in the bones of the face and skull.
Bone Maintenance
Bone is a dynamic tissue
in a constant state of turnover— resorption
and formation. The important regulating factorsthat determine the balance
between bone formation and bone resorption include local stress, vitamin D,
parathyroid hor-mone, calcitonin, and blood supply.
Local stress (weight
bearing) acts to simulate bone formation and remodeling. Weight-bearing bones
are thick and strong. Without weight-bearing or stress, as in prolonged bed
rest, the bone loses calcium (resorption) and becomes osteopenic and weak. The
weak bone may fracture easily.
Biologically active
vitamin D (calcitriol) functions to increase the amount of calcium in the blood
by promoting absorption of calcium from the gastrointestinal tract. It also
facilitates mineral-ization of osteoid tissue. A deficiency of vitamin D
results in bone mineralization deficit, deformity, and fracture.
Parathyroid hormone and
calcitonin are the major hormonal regulators of calcium homeostasis.
Parathyroid hormone regulates the concentration of calcium in the blood, in
part by promoting movement of calcium from the bone. In response to low
cal-cium levels in the blood, increased levels of parathyroid hormone prompt
the mobilization of calcium, the demineralization of bone, and the formation of
bone cysts. Calcitonin, secreted by the thy-roid gland in response to elevated
blood calcium levels, inhibits bone resorption and increases the deposit of
calcium in bone.
Blood supply to the bone
also affects bone formation. With diminished blood supply or hyperemia
(congestion), osteogene-sis (bone
formation) and bone density decrease. Bone necrosisoccurs when the bone is
deprived of blood.
Bone Healing
Most fractures heal through a combination of
intramembranous and endochondral ossification processes. When a bone is
frac-tured, the bone fragments are not merely patched together with scar
tissue. Instead, the bone regenerates itself.
Fracture healing occurs in four areas, including:
·
Bone marrow, where endothelial
cells rapidly undergo trans-formation and become osteoblastic bone-forming
cells
·
Bone cortex, where new osteons
are formed
·
Periosteum, where a hard
callus/bone is formed through intra-membranous ossification peripheral to the
fracture, and where a cartilage model is formed through endochondral
ossification adjacent to the fracture site
·
External soft tissue, where a
bridging callus (fibrous tissue)
stabilizes the fracture
Buckwalter (2000) summarized the process of fracture
healing into six stages stimulated by the release and activation of biologic
regulators and signaling molecules:
·
Hematoma and inflammation: The body’s response is similarto that after injury
elsewhere in the body. There is bleeding into the injured tissue and formation
of a fracture hematoma. The hematoma is the source of signaling molecules, such
as cytokines, transforming growth factor-beta (TGF-β),
and platelet-derived growth factor (PDGF), which initiate the fracture healing
processes. The fracture fragment ends be-come devitalized because of the
interrupted blood supply. The injured area is invaded by macrophages (large
white blood cells), which débride the area. Inflammation, swelling, and pain
are present. The inflammatory stage lasts several days and resolves with a
decrease in pain and swelling.
· Angiogenesis and cartilage
formation: Under the influence ofsignaling molecules, cell
proliferation and differentiation occur. Blood vessels and cartilage overlie
the fracture.
· Cartilage calcification: Chondrocytes
in the cartilage callusform matrix vesicles, which regulate calcification of
the car-tilage. Enzymes within these matrix vesicles prepare the cartilage for
calcium release and deposit.
· Cartilage removal: The
calcified cartilage is invaded byblood vessels and becomes resorbed by
chondroblasts and osteoclasts. It is replaced by woven bone similar to that of
the growth plate.
· Bone formation: Minerals
continue to be deposited untilthe bone is firmly reunited. With major adult
long bone fractures, ossification takes 3 to 4 months.
· Remodeling: The
final stage of fracture repair consists ofre-modeling
the new bone into its former structural arrange-ment. Remodeling may take
months to years, depending on the extent of bone modification needed, the
function of the bone, and the functional stresses on the bone. Cancel-lous bone
heals and remodels more rapidly than does com-pact cortical bone.
Serial x-ray films are used to monitor the progress of
bone healing. The type of bone fractured, the adequacy of blood sup-ply, the
surface contact of the fragments, and the general health of the person
influence the rate of fracture healing. Adequate immobilization is essential
until there is x-ray evidence of bone formation with ossification.
BONE HEALING WITH FRAGMENTS FIRMLY APPROXIMATED
When fractures are
treated with open rigid compression plate fixation techniques, the bony
fragments can be placed in direct contact. Primary bone healing occurs through
cortical bone (Haversian) remodeling. Little or no cartilaginous callus
develops. Immature bone develops from the endosteum. There is an inten-sive
regeneration of new osteons, which develop in the fracture line by a process
similar to normal bone maintenance. Fracture strength is obtained when the new
osteons have become established.
Related Topics