Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Growth Hormones
Protein Manufacture, Formulation and Stability- Growth Hormones
PROTEIN MANUFACTURE, FORMULATION AND STABILITY
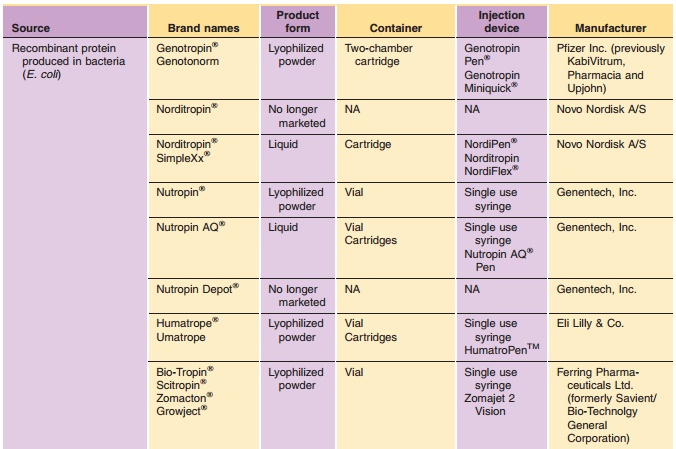
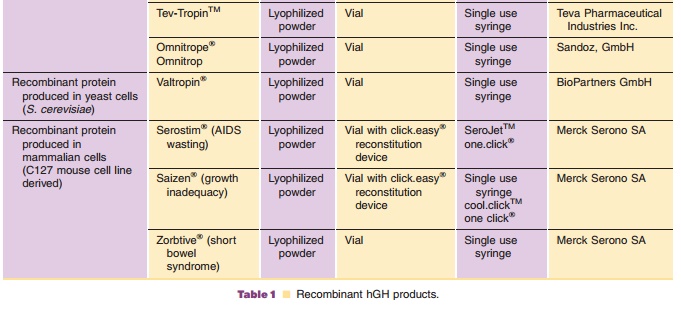
Commercially available hGH preparations are sum-marized in Table 1. All
recombinant growth hor-mones except Serostim / Saizen / Zorbtive are produced
in bacteria or yeast (E. coli, S. cerevisiae). Serostim / Saizen / Zorbtive are
produced in mammalian cells (C127 mouse cells). Growth hor-mone produced in E.
coli may contain an N-terminal methionine residue. Natural sequence rhGH is
produced either by enzymatic cleavage of the methionine during the purification
procedure or by periplasmic secretion of rhGH into refractile bodies. The rhGH
is then released from the refractile bodies by osmotic shock and the protein
recovered and purified. rhGH synthesized in mammalian cells is transported
across the endoplasmic reticulum and secreted directly into the culture medium
from which it is recovered and purified.
Historically, the potency of hGH products was expressed in international
units per mg (IU/mg). The initial standard, established in 1982 for pit-hGH
preparations, was 2 IU/mg. The standard for rhGH products was 2.6 IU/mg until
September 1994. The current WHO standard, established in September 1994, is 3.0
IU/mg. Dosages are usually expressed as IU/kg or IU/m2 in Europe and Japan and as mg/kg
in the United States. However, the use of IU dosages is no longer necessary due
to the high level of purity and consistent potency of recombinant hGH products.
All current rhGH products are available as lyophilized or liquid
preparations. Lyophilized for-mulations usually include 5 or 10 mg of protein
in a glycine and mannitol or sucrose-containing phos-phate buffer excipient.
The materials are usually reconstituted with sterile water for injection for
single use or with bacteriostatic water or bacteriostatic saline for multiple
injection use. Liquid formulations of rhGH (Nutropin AQ , Norditropin ,
SimpleXx ) contain mannitol or sodium chloride, histidine or citrate buffer,
poloxamer 188 or polysorbate 20 and phenol. Product stability has been very
good with shelf lives of approximately 2 years at 2 C to 8 C. A long-acting
dosage form of rhGH (Nutropin Depot ) was available from 1999 until 2004.
Nutropin Depot contained micronized particles of rhGH embedded in
biocompatible, biodegradable polylactide-coglycolide (PLGA) microspheres.
Omnitrope /Omnitrop (US/EU) and Valtropin (EU only), lyophilized rhGH preparations,
were approved for marketing as the first “biosimilar” rhGH products in 2006.
Related Topics