Chapter: Aquaculture Engineering : Disinfection
Ozone - Disinfection in Aquaculture Engineering
Ozone
Function
Ozone (O3) is a colourless gas with a boiling point of −112°C, that is sometimes called trioxygen.
Ozone gas is unstable and will quickly be broken down to O2; the half-life of O3 is around 15 minutes. It is therefore necessary to produce the ozone on site.
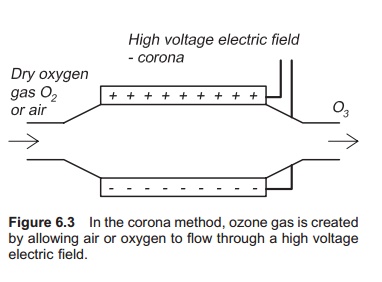
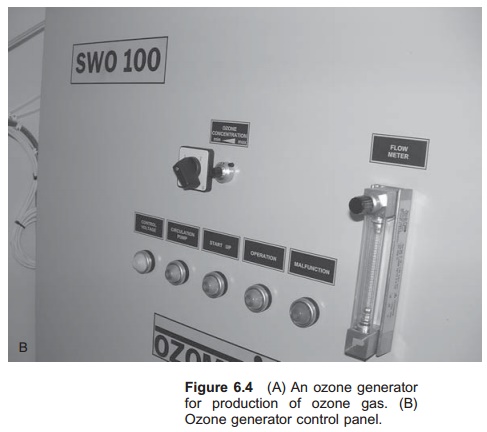
Ozone is produced by the corona method; air or pure oxygen gas is passed through a high voltage electric field (Fig. 6.3). This is actually the same process that happens with lightning in a thunder-storm, where ozone gas can also be smelled. Energy is added to the oxygen molecule and ozone is created: 3O2+ energy = 2O3. An ozone generator is shown in Fig. 6.4, which can use either pure oxygen or air to produce ozone. If using air, for the highest cost effectiveness, it must be as dry as possible before entering the ozone generator. An air drier must therefore be used. If using air, the water may become super-saturated with nitrogen. Using pure oxygen to generate ozone is more expensive. The best source of oxygen to use must be decided on a case by case basis. Use of air result in 0.5–3% ozone in the gas stream, whereas the corresponding values for oxygen are 1–6%. Energy requirements for producing ozone are typically in the range 3–30 kWh/kg.7,11 Only a small amount of (5–10%) the supplied energy is used for ozone production. Small quantities of ozone may also be produced by radiation of air or oxygen gas with UV light of specific wavelength; this is named photozone.

Mode of action
Ozone is a very strong oxidizing agent, highly toxic to all forms of life. When dissolved in water it starts two reactions, slow direct oxidation of organic substances by O3and a chain reaction with formation of different free radicals based on hydroxyl radical (OH−). Ozone acts by damaging cell mem-branes and nucleic acids, breaking long chain molecules down into simpler forms which may be further degraded in the biological filter. This inacti-vates the micro-organisms. Ozone has another effect that could be an advantage in aquaculture systems: by oxidation it reduces the amount of NH3, NO2 and BOD biofilm on surfaces. This can be seen in water re-use systems where disinfection with ozone improves water quality and may therefore be more beneficial than use of other disinfectants. Oxidation by ozone eliminates the yellow/brown coloration of the water that is built up in a water re-use system with very high degrees of re-use. When using ozone as a disinfectant it is recommended that particles be removed from the water before the ozone is added, otherwise much of the ozone will be used to oxidize the particles.
Design specification
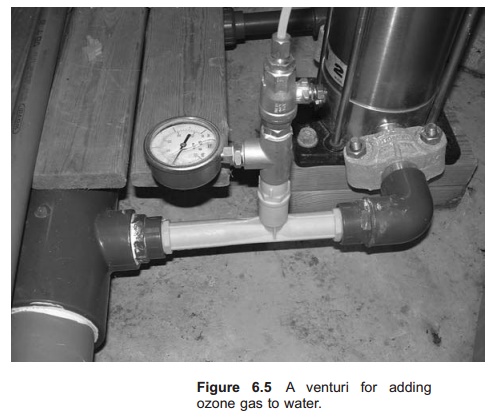
When adding ozone gas to water a special injection system has to be used to ensure good gas water mixing. The method is similar to those for mixing oxygen gas into water and use of a venturi is quite common (Fig. 6.5), 90% transfer of ozone has been achieved with a properly designed diffuser system.4 An ozone disinfection system therefore consists of a production system, the generator and an injection system.
Since there is a dose–time relation for ozone dis-infection, the ozone needs to have a certain working time in the water to function and oxidize the micro-organisms. A water retention tank is quite com-monly used. Ozone is added to the water which then enters the retention tank. This must be large enough to ensure a satisfactory contact time for the ozone to achieve disinfection. Alternatively, the ozone can be added at the beginning of the inlet pipe to the aquaculture plant. Because the water takes some time to reach the fish tanks, this might be enough to achieve sufficient contact between ozone and the micro-organisms to disinfect the water.
When designing an ozone disinfection plant, it is necessary to include an injection system to get the ozone gas into the water and a system that gives sufficient retention time between the ozone and the water. It is important that the residual concentra-tion of ozone is above the value that is needed for disinfection, this is, of course, less than the inlet concentration.
Ozone dose
To inactivate pathogens with ozone a dose–time relation applies, as for many other disinfectants.
Either a high dose can be used for a short time, or vice versa. Over-dosing must be avoided because this may kill the fish.
Example 3
Ozone is being used to inactivate the bacterium Vibrio anguillarum. The dose is set to 0.1 mg/(l min)for a log disinfection of 3. This can be achieved by having a residual concentration of 0.1 mg/l after 1 minute working time or 0.5 mg/l after 2 minutes.
Most pathogens are killed by an ozone dose of 0.1–1.0 mg/l and contact time of 1–10 minutes, but this varies with the organism.
The water quality will have large impact on the residual ozone concentration after a given time; factors such as concentration of dissolved organics, particular organics, inorganic ions, pH and temper-ature will affect the concentration (for example, see refs 1, 13). An increase in temperature results in a reduced lethal dose. Because of these variations it is important to add enough ozone to obtain a satisfactory residual concentration to achieve disinfection.
Special problems
The great problem with the use of ozone is that it is highly toxic for fish and humans. For fish, ozone is toxic even at relatively small concentrations, because it oxidizes the gill tissue. Recommended safe values for fish are generally below 0.002 mg/l, but there are large variations in tolerance.14 There-fore after adding ozone to the water and leaving it to react for the necessary time, any residual ozone must be removed or destroyed. Having an adequate retention time ensures that most of the ozone has reacted and the product is mainly oxygen gas (O2): this time is normally much longer than that neces-sary to achieve satisfactory disinfection, but of course depends on the decomposition rate. The retention time must therefore be long enough for two processes: (1) to get the ozone to destroy the micro-organisms and (2) to remove residual ozone toxic to the fish. Non-toxic ozone concentra-tions are normally achieved after 10–20 minutes. This can be done either by increasing the size of the retention tank or by increasing the rate of ozone decomposition to oxygen; methods here include aeration of the retention tank, ozone removal by sending the water through a carbon filter, stripping of the water in a packed column and addtion of chemicals.
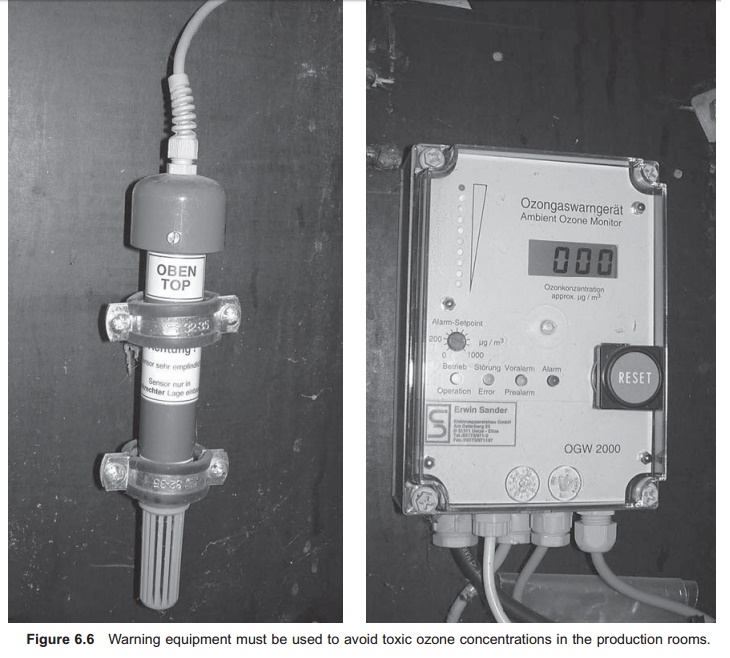
Ozone is also toxic to humans, even a very low concentration of ozone in the air being harmful. In the USA, the maximum during an 8-hour work shift in an enclosed area is set to 0.1 mg/l O3; for 10 minutes exposure time the maximum level is 0.2 mg/l.2 Humans can detect levels of 0.02–0.05 mg/l by the smell, so when ozone is smelt the build-ing must be evacuated immediately. The possibili-ties of ozone gas entering the air which humans breathe must therefore be minimized. Proper ven-tilation in the room where the ozone is produced and added is absolutely essential. In addition there must be safety equipment to monitor the concen-tration of ozone in the air continuously, and equip-ment that automatically turns off the ozone supply if the concentration rises above the recommended values; in addition, warning signals must be given (Fig. 6.6).
Another problem with the use of ozone results from it being a very strong oxidizing agent. It is so effective that it will oxidize all materials with which it comes into contact, whether as a gas or dissolved in water. Plastic and metals are examples of mate-rials that the ozone gas will try to oxidize. Ozone will destroy most plastics to various extents. Polypropylene pipes are recommended for trans-porting water with a high content of ozone. Ozone will oxidize metals, causing significant corrosion problems. Special additives must be used in fibre-glass if it is to be used in retention tanks for ozonated water, for instance.
Measuring ozone content
Control of the content of ozone is essential: enough must be injected but overdosing avoided to prevent harm to the fish and to humans as a result of degassing of ozone to the air in an enclosed space. The ozone content of water can be measured either by a chemical method employing indigo triosulfonate or by using a probe for online measurement employing a potentiometric principle. However, online equipment is quite expensive. Measurement of the redox potential which changes with the amount of ozone in the water is therefore quite commonly used instead.
Related Topics