Chapter: Microbiology and Immunology: Virology, Virus: Laboratory Diagnosis of Viral Diseases
Methods of Laboratory Diagnosis of Viral Diseases
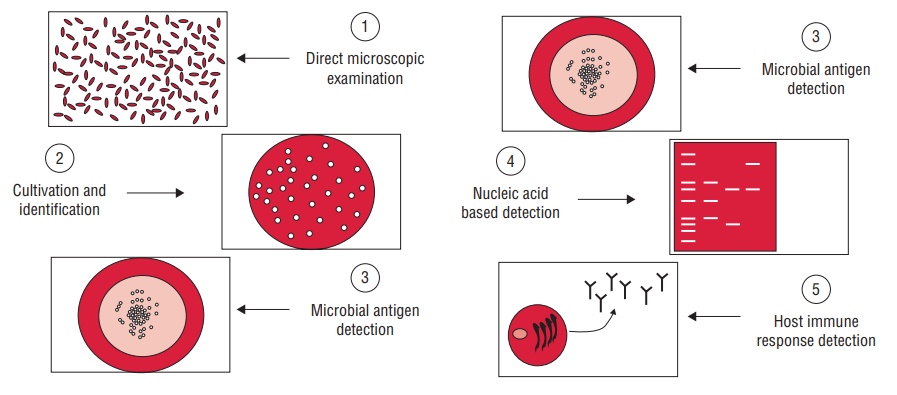
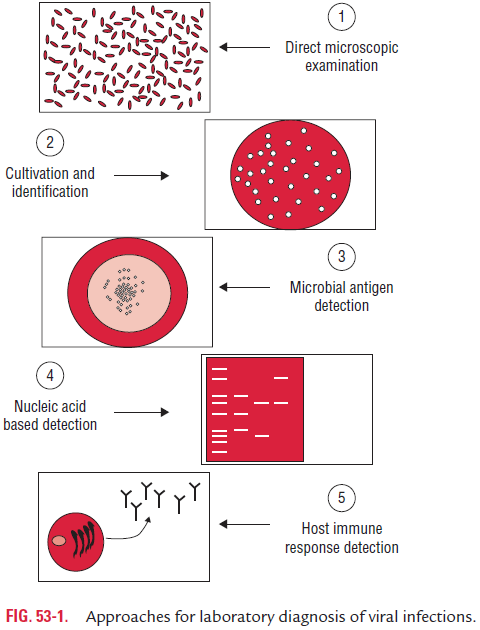
Methods of Laboratory Diagnosis
Laboratory diagnosis of viral infections can be carried out by many methods. These methods include
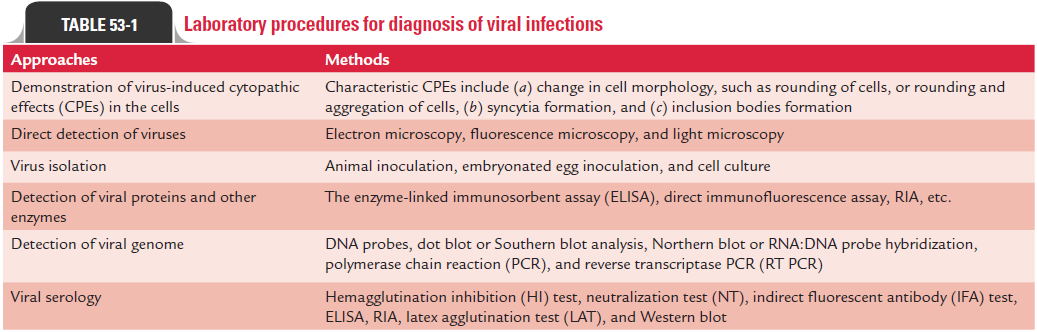
(a) demonstration of virus-induced cytopathic effects (CPEs) in the cells,
(b) direct detection of viruses,
(c) virus isolation and viral assays,
(d) detection of viral proteins and other enzymes,
(e) detection of viral genome, and
(f) viral serology (Fig. 53-1).
Demonstration of Virus-Induced CPEs in the Cells
Many viruses produce characteristic morphological changes in the cells they infect. These changes are known as CPEs, and the viruses causing the CPEs are known as cytopathogenic viruses. The characteristic CPEs include (a) change in cell morphology, such as rounding of cells or rounding and aggregation of cells, (b) syncytia formation, and (c) inclusion bodies formation.
· Replication of virus in infected cells may cause rounding, refractility, degeneration, and nuclear pyknosis. This may finally lead to complete or partial cell lysis as well as vacuolation, as seen in picornaviruses. Viruses, such as adenoviruses, may cause rounding of cells and aggregate to form grape-like clusters.
· Viruses, such as paramyxovirus, varicella zoster, respiratory syncytial virus, and herpes simplex virus (HSV), cause for-mation of syncytia containing several (up to 100) nuclei in infected cells. These syncytia are multinucleated giant cells formed by fusion of virus-infected cells with neighboring or uninfected cells.
· Inclusion bodies are intranuclear or cytoplasmic bodies pro-duced by viruses in infected cells. They are produced as a result of histological changes in infected cells caused by viral components. These are also produced as a result of virus-induced changes in cell structures.
These inclusion bodies can be demonstrated on staining by light microscopy. These inclusion bodies may be acidophilic or basophilic, small or large, round or irregular; and single or multiple. These bodies may be present either in nucleus or cytoplasm, or in both. Many viruses produce different types of inclusion bodies. For example, rabies virus produces intracyto-plasmic inclusion bodies called Negri bodies in neural tissue of the brain. Cytomegalovirus (CMV) produces intranuclear owl’s eye inclusion bodies (Color Photo 53) in infected cells or in cell sediments excreted in the urine of the patient infected with CMV. Human herpes viruses produces Cowdry type A inclusion bodies. Measles virus produces inclusion bodies that are seen both in the cytoplasm and nucleus of infected cells (Table 53-1).
Direct Detection of Viruses
Viruses can be detected directly in clinical specimens by elec-tron microscopy (EM), fluorescence microscopy, and light microscopy.
◗ Electron microscopy
Electron microscopy is the most commonly used method for direct detection of virus in clinical specimens for diagnosis of many viral diseases. EM can be used to detect distinctive viruses or viral structures directly in appropriate clinical specimens or in biopsy for diagnosis of viral infections. EM is not a routinely used test for detection of viral infection and is performed only in the laboratories equipped with electron microscope.
◗ Fluorescence microscopy
Direct fluorescence microscopy (DFA), using specific monoclo-nal or polyclonal antibody, is employed to detect viral antigens on the cell surface or within the cells infected by viruses. The viral antigens can be detected (a) in the acetone-fixed cell smears, (b) in the frozen tissue sections of the cells from virus-infected cells, and (c) also in vesicular fluid. The DFA test has been employed for:
· antemortem diagnosis of rabies by detection of rabies-virus antigen in smears from the face or nape of the neck;
· demonstration of viral antigens in brain biopsy specimens for diagnosis of herpes simplex encephalitis and subacute sclerosing encephalitis; and
· rapid diagnosis of infections caused by adenoviruses, paramyxoviruses, and orthomyxoviruses.
◗ Light microscopy
Viral antigens in infected cell cultures are demonstrated by immunoperoxidase staining.
Virus Isolation
Demonstration of virus in appropriate clinical specimens by culture establishes diagnosis of viral diseases. Isolation of virus is always considered as a gold standard for establishing viral etiology of a disease. Collection of appropriate clinical speci-mens depends on type of the viral disease. For example, cere-brospinal fluid (CSF) is the specimen of choice for diagnosis of viral infections of the central nervous system (CNS) caused by arboviruses, picornavirus, or rabies virus. Blood is the speci-men frequently examined for diagnosis of HIV and hepatitis B, C, and D infections and other blood-borne viral infections. The tests of other clinical specimens to be collected for diagnosis of other types of viral diseases are summarized in Table 53-2.
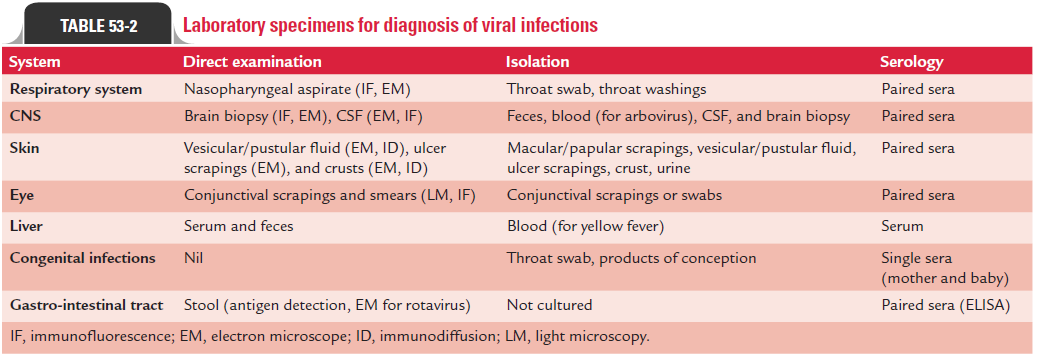
Timing of collection of specimen is important. The speci-mens collected early in the acute stage infection—before the shedding of virus is stopped—are most important. For exam-ple, enteroviruses are present in the CSF for only 2–3 days after the onset of CNS manifestations. Herpes simplex virus and varicella zoster virus are found in lesions only within first 5 days of onset of symptoms and respiratory viruses are present in respiratory secretions during only first 3–7 days of onset of symptoms.
Immediate transport of the specimen to laboratory for pro-cessing also facilitates better isolation of virus from clinical specimens. The viruses are usually heat labile, and the clini-cal specimens may be infected secondarily by contamination with bacteria and fungi. Hence, clinical specimens for viruses are usually transported and stored on ice. They are transported in special transport media that contain antibiotics to inhibit bacterial and fungal contaminants and also contain proteins, such as serum albumin or gelatin. Some of the viruses, such as influenza virus, HSV, and VZV lose their infectious titer when clinical specimens are stored at room temperature or kept fro-zen at220°C. Most of the viruses can be cultivated in (a) exper-imental animals, (b) embryonated eggs, or (c) tissue culture.
◗ Animal inoculation
Mouse is most frequently used for isolation of viruses by animal inoculation. In addition, rabbits, hamsters, newborn or suckling rodents are also used. Experimental animals are rarely used for cultivation of viruses but play an essential role in study of pathogenesis of viral infections and that of viral oncogenesis.
Intracerebral, subcutaneous, intraperitoneal, or intranasal routes are various routes of inoculation. After inoculation, the animals are observed for signs of disease or death. The infected animals are then sacrificed and infected tissues are examined for the presence of viruses by various tests, and also for inclusion bodies in infected tissues. Furthermore, infant (suckling) mice are used for isolation of coxsackie virus and rabies virus.
◗ Embryonated eggs
Embryonated eggs were used initially for the growth of viruses. Embryonated chick egg was used first for cultivation of viruses by Goodpasture in 1931. The method further developed by Burnet was used for cultivation of viruses in different sites of the embryonated egg. Usually, 8–11 days’ old chick eggs are used for culture of viruses. The viruses are isolated in different sites of the egg, such as yolk sac, amniotic cavity, and allantoic cavity, and chorioallantoic membrane (CAM) (Fig. 53-2).
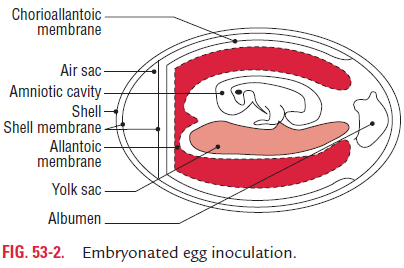
Many of these viruses cause well-defined and characteris-tic foci, providing a method for identification, quantification, or assessing virus pathogenicity. The embryonated egg is also used for growing higher titre stocks of some viruses in research laboratories and for vaccine production.
Yolk sac: Yolk sac inoculation is used for cultivation of Japaneseencephalitis, Saint Louis encephalitis, and West Nile virus. It is also used for growth of chlamydia and rickettsia.
Amniotic cavity: Inoculation in the amniotic cavity is usedmainly for primary isolation of influenza virus.
Allantoic cavity: Inoculation in the allantoic cavity is used forserial passages and for obtaining large quantities of virus, such as influenza virus, yellow fever (17D strain), and rabies (Flury strain) viruses for preparation of vaccines. For production of rabies virus, duck eggs were used due to their bigger size than that of hen’s egg. This helped in production of large quanti-ties of rabies virus, which are used for preparation of the inacti-vated non-neural rabies vaccine.
Chorioallantoic membrane: Inoculation of some viruseson CAM produced visible lesions known as pocks. Each infec-tious virus particle produces one pock. The pox viruses, such as variola or vaccinia are identified by demonstration of typical pocks on the CAM inoculated with the pox virus. Nowadays, in a virology laboratory, chick embryo inocula-tion has been replaced by cell cultures for routine isolation of viruses.
◗ Tissue culture
Cell culture is most widely used in diagnostic virology for culti-vation and assays of viruses. The tissue culture was first applied in diagnostic virology by Steinhardt and colleagues in 1913. They maintained the vaccinia virus by culture in tissues of rabbit cornea. Subsequently, Maitland (1928) used cut tissues in nutrient media for cultivation of vaccine viruses. Enders, Weller, and Robins (1949) were the first to culture poliovirus in tissue cultures of nonneural origin. Since then, most of the virus had been grown in tissue culture for diagnosis of viral dis-eases. Different types of tissue cultures are used to grow viruses. Tissue culture can be of three different types as follows:
Organ culture
This was used earlier for the isolation of some viruses, which appear to show affinity for certain tissue organs. For exam-ple, coronavirus, a respiratory pathogen, was isolated in the tracheal ring organ culture. In this method, small bits of the organs are maintained in vitro for days and weeks preserving their original morphology and function. Nowadays, organ cul-ture is not used.
Explant culture
In this method, components of minced tissue are grown as explants embedded in plasma clots. Earlier, adenoid tissue explant cultures were used for isolation of adenoviruses. This method is now seldom used in virology.
Cell culture
Cell culture is now routinely used for growing viruses. In this method, tissues are dissociated into component cells by treat-ment with proteolytic enzymes (trypsin or collagenase) followed by mechanical shaking. The cells are then washed, counted, and suspended in a growth medium containing essential amino acids and vitamins, salts, glucose, and a buffering system. This medium is supplemented by up to 5% of fetal calf serum and antibiotics. The cell suspension is dispensed in glass or plastic bottles, tables, or Petri dishes. On incubation, the cells adhere to the glass surfaces and divide to form a confluent monolayer sheet of cells covering the surface within a week (Fig. 53-3). The cell culture may be incubated either as a stationery culture or as a roller drum culture. The latter is useful for growth of some fastidious viruses due to better aeration by rolling of the
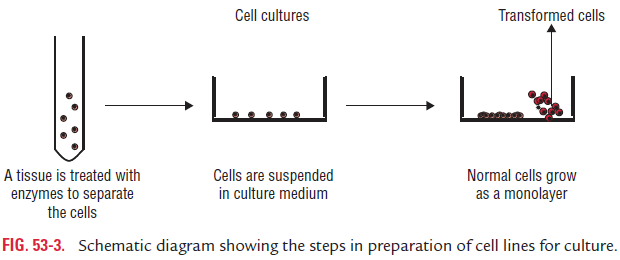
culture bottle in special roller drums. The cell cultures are clas-sified into three different types based on their origin, chromo-somal characters, and number of generations for which they can be maintained, as follows (Table 53-3):
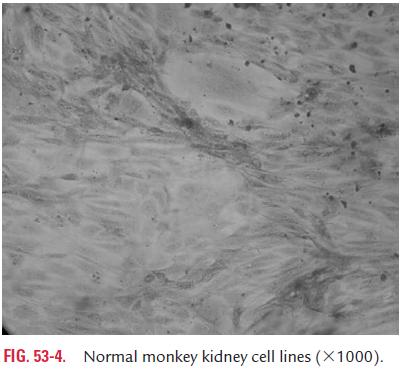
Primary cell culture: These are a culture of normal cellsobtained freshly from the original tissues that have been cultivated in vitro for the first time and that have not been subcultured. These cell cultures can be established from whole animal embryo or from selected tissues from adult, newborn, or embryos. These cells have the normal diploid chromosomal number and are capable of only limited growth (5–10 divisions) in culture. They cannot be maintained in serial culture, but can be subcultured to obtain large number of cells. Monkey kidney cell culture (Fig. 53-4, Color Photo 54), human embryonic kidney cell culture, and chick embryo cell culture are the common examples of primary cell culture. Primary monkey kidney cell cultures are highly useful for the primary isolation of myxovirus, paramyxovirus, many enteroviruses, and some adenoviruses.
Diploid cell strains: Diploid cell strains are of a single celltype that retains their original diploid chromosome number and karyotype. However, they have specific characteristics and compositions and are usually composed of one basic cell type.
They are usually fibroblasts and can be cultured for maximum 50 serial passages before they undergo senescence (die off) or undergo a significant change in their characteristics. Diploid cells derived from human fibroblasts are useful for isolation of some fastidious viruses. They are also used for production of vaccines; for example, WI-38 human embryonic lung cell stem is used for the cultivation of fixed rabies virus, and human fetal diploid cells for isolation of adenovirus, picornaviruses, HSV, CMV, and VZV.
Continuous cell lines: Continuous or immortal cell lines arecells of a single type, which are derived from cancerous tissue and are capable of continuous serial cultivation indefinitely without senescing. The cells are usually derived from diploid cell lines or from malignant tissues and have altered and irregular number of chromosomes. Immortalization may occur spontaneously or can be induced by chemical mutagens, tumorigenic viruses, or oncogens. Hep-2, HeLa, and KB derived from human carcinoma cervix, human epithelioma of larynx, and human carcinoma of nasopharynx and other cell lines are excellent for recovery of a large number of viruses. These cell lines have been used extensively for the growth of a number of viruses. These cell lines are usually stored at 270°C for use when necessary or are maintained by serial subculture. The type of cell line used for virus culture depends on the sensitivity of the cells to a particular virus; for example, Hep-2 cell line is excellent for the recovery of respiratory syncytial viruses, adenoviruses, and HSV. Most of the viruses can be isolated by using one of these cell lines. Growth of viruses in cell cultures can be detected by the following methods:
· Cytopathic effect :Many viruses can be detected and initiallyidentified by observation of the morphological changes in the cultured cells in which they replicate. The CPE pro-duced by different types of viruses are characteristic and help in the initial identification of virus isolates. Nuclear shrinking, vacuoles in the cytoplasm, syncytia forma-tion, rounding up, and detachment are the examples of alteration of morphology of the cells. Most CPEs can be demonstrated in unfixed and unstained monolayer of cells under low power of microscope. For example, adenoviruses produce large granular changes resembling bunches of grapes, SV-14 produces well-defined cytoplasmic vacuola-tion, measles virus produces syncytium formation, herpes virus produces discrete focal degeneration, and enterovi-ruses cause crenation of cells and degeneration of the entire cell sheet.
· Hemadsorption:Hemadsorption is the process of adsorp-tion of erythrocytes to the surfaces of infected cells which serves as an indirect measurement of viral protein synthe-sis. This property is made use of to detect infection with noncytocidal viruses as well as the early stage of cytocidal viruses. Viruses, such as influenza virus, parainfluenza virus, mumps virus, and togavirus, when infect cell lines code for the expression of red cell agglutinins, which are expressed on the infected cell membrane during infections. These hem-agglutinins bind some erythrocytes to the infected cell sur-face. Sometimes, viruses can be detected by agglutination of erythrocytes in the culture medium.
· Heterologous interference:This property is used to detectviruses that do not produce classic CPEs in the cell lines. In this method, the growth of non-CPE-producing virus in cell culture can be tested by subsequent challenge with a virus known to produce CPEs. The growth of the first virus will inhibit infection by the cytopathic challenge virus by inter-ference. For example, rubella virus usually does not produce any CPE, but prevents the replication of picornaviruses, which is inoculated as a cytopathic challenge virus.
·
· Transformation:Oncogenic viruses that are associated withformation of tumors induce cell transformation and loss of contact inhibition in the infected cell lines. This leads to surface growth that appears in a piled-up fashion producing microtumors. Examples of such oncogenic viruses that pro-duce transformation in cell lines are some herpes viruses, ade-noviruses, hepadanoviruses, papovavirus, and retroviruses.
· Light microscopy:Viral antigens in infected cell cultures aredemonstrated by staining virus-infected cells of tissue sec-tions with specific viral antibody conjugated with horse-radish peroxidase. This is followed by addition of hydrogen peroxide along with a benzidine derivative substance. In a positive reaction, a red insoluble precipitate is deposited on the cell line, which is demonstrated by examination under ordinary light microscope.
· Immunofluorescence:Direct immunofluorescence usingspecific antibodies is frequently used to detect viral antigens in inoculated cell lines for identification of viruses.
· Electron microscopy:The viruses can also be demonstrated ininfected cell lines by EM.
Viral Assays
The virus content of a specimen can be assessed either by estimating total virus particles count or by assay of infectivity of viruses.
◗ Total virus particles count
Electron microscopy and hemagglutination are the two meth-ods used for estimation of total virus particles.
Electron microscopy: EM is useful to count virus particlesdirectly in a negatively stained viral suspension. In this method, the virus suspension is mixed with a known concentration of latex particles, and the number of virus particles in the suspen-sion is estimated by a ratio between the virus and latex particles demonstrated by EM.
Hemagglutination: Quantitation of hemagglutinating virusesis carried out by determination of hemagglutination titers. Although it is not a sensitive method, it is used as a convenient method of virus assay. For example, approximately, 107 influ-enza virions are essential to produce microscopic agglutination in cultured cells.
◗ Assay of infectivity of viruses
Quantitative and quantal assays are the two types of assays, which are carried out to determine assay of infectivity of viruses.
Quantitative assay of infectivity: Quantitative assay is usedto estimate the presence of actual number of viable infectious viral particles in the inoculum. Two methods are available for the purpose, which include plaque assay in monolayer cell culture and pock assay on chick embryo CAM.
· Plaque assay: It was introduced by Dulbecco in 1952 as amodification of bacteriophage plaque assay. This is based on the principle that each infectious viral particle gives rise to a localized focus of infected cells that can be visual-ized by the naked eye. Such foci are called plaques, and each plaque indicates an infectious virus. The test is performed by adding a viral inoculum to a monolayer of culture cells in a bottle or Petri dish. After sometime, this allows adsorp-tion of viruses. The liquid medium is removed and replaced with a solid agar gel to ensure that the spread of progeny virus is confined to the immediate vicinity of infected cells.
· Pock assay: Viruses that form pocks on CAM can beassayed by counting the number of pocks formed on the inoculated CAM. Each pock on CAM arises from a single virus particle. This is known as pock assay. Vaccinia and variola viruses can be assayed by pock assay.
Quantal assays of infectivity: Quantal assays of infectivitycan be carried out to quantitate a virus by quantitating the virus particles in animals, in embryonated eggs, or in tissue culture. This method of assay of infectivity only indicates the presence or absence of infectious viruses, but it does not indi-cate actual number of viruses. The endpoints used for infec-tivity titrations are estimated by the (a) development of CPE in cell cultures, (b) production of hemagglutination in allan-toic fluid of embryonated egg, or (c) death of experimentally infected animals. The titer of virus is usually expressed as the “50% infectious dose (ID50)” per mL, which indicates the high-est dilution of the inoculum that initiates detectable symp-toms, antibodies, or other responses in 50% of inoculated test animals, eggs, or cell cultures.
Detection of Viral Proteins and Other Enzymes
During replication of viruses in host cells, viral proteins, antigens, and other enzymes are produced. These viral products and com-ponents can be detected by many methods including biochemical, immunological, and molecular methods in clinical specimens.
Enzyme-linked immunosorbent assay (ELISA), direct immu-nofluorescence assay, and radioimmunoassay (RIA) are widely used methods. These methods are used for detection and identi-fication of viruses and viral antigens in clinical specimens, as well as in cell cultures. These tests use specific monoclonal or mono-specific antibodies that are raised against specific viral antigens.
Identification and quantification of specific virus can also be carried out by detection and assay of characteristic viral enzymes. For example, detection of reverse transcriptase in serum or cell culture suggests the presence of a retrovirus. Similarly, demonstration of hemagglutinins or hemadsorption in the cell culture indicates the presence of influenza virus.
Detection of Viral Genome
The unique genomic structure and genetic sequences are the most important characteristics of the type and family of virus. Therefore, recently molecular methods are increasingly used for diagnosis of viral diseases. The restriction endonucle-ase fragment lengths from the genome of DNA viruses, such as HSV-1 and HSV-2 or the electrophoretic pattern of RNA viruses, such as influenza and reo viruses are considered as genetic fingerprints for these viruses.
The methods for detection of viral genome include (a) DNA probes, (b) dot blot or Southern blot analysis, (c) Northern blot or RNA:DNA probe hybridization, (d) polymerase chain reaction (PCR), and (e) reverse transcriptase PCR (RT PCR).
Viral Serology
Viral serology is based on detection of specific viral antibod-ies in serum of the infected human host. A wide number of serological tests are used for demonstration of specific viral antibodies in patient’s sera. These include hemagglutination inhibition (HI) test, neutralization test (NT), indirect fluores-cent antibody (IFA) test, ELISA, RIA, latex agglutination test (LAT), and Western blot.
The detection of virus-specific immunoglobulin (IgM) antibody in serum indicates a recent primary infection. This is because IgM antibodies appear in the serum during first 2 or 3 weeks of primary infection. A fourfold increase in the antibody titer between the serum collected during the acute phase of the disease and during the convalescent phase, 2–3 weeks after acute phase, is suggestive of seroconversion. An anamnestic or secondary antibody response occurs during reinfection or recurrence of viral infection later in life. The serum antibody titer usually remains high in individuals who suffer frequent recurrence of the disease, such as herpes virus.
· HI test is used for detection of viruses that agglutinate red blood cells of chickens, guinea pigs, human, or other mammals. Antibodies present in serum prevent viruses to bind and to agglutinate the new blood cells. The HI test is used for diagnosis of infections caused by orthomyxoviruses.
· NT is based on inhibition of infection by the antibody and that of CPEs of the viruses in tissue culture cells. The neu-tralization antibody response is usually virus and strain spe-cific. The highest dilution of serum preventing infectivity is 50% of virus. Serum mixture tested is considered as the titer of the test.
· ELISA is most commonly used for screening of blood samples for hepatitis B and C viruses and HIV.
· IFA, RIA, and LAT are also used for diagnosis of many common viral infections.
· Western blot is most commonly used to confirm infection caused by HIV, initially diagnosed by ELISA.
The limitations of serological tests in viral diseases are the following:
· The presence of antiviral antibody in serum only indicates infection but cannot determine whether it is recent or old. Demonstration of IgM antibodies or demonstration of a fourfold increase in the antibody titer between acute and convalescent sera indicates only recent infection.
· The serological tests may be associated with false-positive or false-negative reactions. The serological cross-reaction may occur between different viruses, giving rise to false-positive reactions. Formation of immune complexes in serum may give rise to false-negative reaction as observed in viral infection caused by hepatitis B virus.
Related Topics