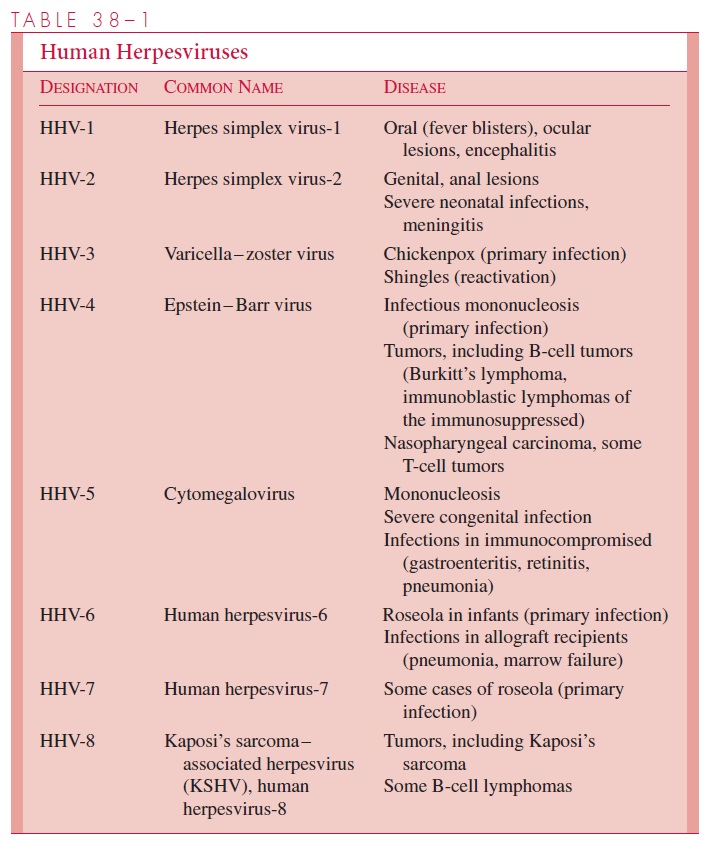
Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Herpesviruses
Herpesviruses - Virology
VIROLOGY
All herpesviruses are morphologically similar, with an overall size of 180 to 200 nm. The DNA core is up to 75 nm in diameter and is surrounded by an icosahedral capsid. Over the capsid is a protein-filled region called the tegument. The outside of the viral particle is covered by a lipoprotein envelope derived from the nuclear membrane of the infected host cell. The envelope contains at least nine glycoproteins that protrude beyond it as spike-like structures. The viral genome is large, up to 240 kbp of DNA, which code for approximately 75 viral proteins. This large genome is necessary, because herpesviruses frequently infect nondividing cells and must therefore provide their own enzymes neces-sary for DNA synthesis. Despite the morphologic similarity between herpesviruses, there are substantial differences in their genomic sequences and, in turn, their structural glyco-proteins and polypeptides. Antigenic analysis is an important means for differentiation among herpesviruses despite some cross-reactions (eg, between HSV and VZV).
Based on certain virologic similarities, the herpes viruses may be divided into three subfamilies α,β , and γ. Herpes simplex 1 and 2, as well as varicella-zoster viruses, are in the subfamily; cytomegalovirus, HHV-6, and HHV-7 are in the subfamily while EBV and HHV-8 are in the γ subfamily.
Cell tropisms for the individual viruses vary significantly. Herpes simplex virus has the widest range; it replicates in numerous animal and human host cells, although it affects only humans in nature. VZV infects only primates and is best grown in cells of human ori-gin, although some laboratory-adapted strains can grow in primate cell lines. Human CMV replicates well only in human diploid fibroblast cell lines. EBV does not replicate in most commonly used cell culture systems but can be grown in continuous human or primate lymphoblastoid cell cultures. Human HSV-6 grows only in lymphocyte cell cultures.
Characteristically, all of these agents produce an initial infection followed by a period of latent infection in which the genome of the virus is present in cells, but infectious virus is not recovered. During latent infection of cells, viral DNA is maintained as an episome (not integrated), with limited expression of specific virus genes required for the mainte-nance of latency. Reactivation of virus due to complex host–virus interactions may then result in recurrent disease. For example, immunocompromised patients, especially those with altered cellular immunity, have frequent reactivations of herpesviruses that can lead to clinically severe disease.
Replication
The replication of HSV is representative of all herpesviruses. The glycoproteins in the HSV envelope interact with cellular receptors to result in fusion with the cell membrane. Fusion delivers the capsid and DNA case into the cytoplasm, where it migrates to the nu- cleus, and the genome is circularized. Transcription of the large, complex genome is se-quentially regulated in a cascade fashion. Three distinct classes of mRNAs are made: (1) immediate early (IE) mRNAs are synthesized 2 to 4 hours postinfection, which code for proteins initiating and regulating virus transcription; (2) early (E) mRNAs, which code for further nonstructural proteins involved in DNA replication and minor structural pro-teins; and (3) late (L) mRNAs (ie, 12 to 15 hours postinfection), which code for major structural proteins. The early (E) proteins include thymidine kinase and a DNA poly-merase, which are distinct from host cell enzymes and are therefore important targets of antiviral chemotherapy. Gene expression is coordinated (ie, synthesis of early gene prod-ucts turns off IE products and initiates genome replication); some of the late structural proteins are produced independently of genome replication, whereas others are only pro-duced after replication. The pattern of viral DNA replication is complex, resulting in the formation of high-molecular-weight DNA concatemers. Genomic concatemers are cleaved and packaged into preassembled capsids in the nucleus.
The envelope is acquired from the inner lamella of the nuclear membrane. Budding occurs at the inner nuclear membranes, and virions then enter the cytoplasm to be re-leased through the endoplasmic reticulum. HSV infection appears to be a “wasteful” process: only 25% of viral DNA/protein produced is incorporated into virions. The rest accumulates in the cell, which eventually dies. Moreover, the ratio of incomplete to com-plete viral particles is approximately 1000 to 1. Most herpesviruses shut down host cell metabolism and ultimately cause cell death, except for CMV, which actually stimulates cellular synthesis of nucleic acids and proteins.
Related Topics