Chapter: Medical Physiology: Protein Metabolism
Functional Roles of the Plasma Proteins
Functional Roles of the Plasma Proteins
The major types of protein present in the plasma are albumin, globulin, and fibrinogen.
A major function of albumin is to provide colloidosmotic pressure in the plasma, which prevents plasmaloss from the capillaries.
The globulins perform a number of enzymatic func-tions in the plasma, but equally important, they are prin-cipally responsible for the body’s both natural and acquired immunityagainst invading organisms.
Fibrinogen polymerizes into long fibrin threadsduring blood coagulation, thereby forming blood clots that help repair leaks in the circulatory system.
Formation of the Plasma Proteins. Essentially all thealbumin and fibrinogen of the plasma proteins, as well as 50 to 80 per cent of the globulins, are formed in the liver. The remainder of the globulins are formed almost entirely in the lymphoid tissues. They are mainly the gamma globulins that constitute the antibodies used in the immune system.
The rate of plasma protein formation by the liver can be extremely high, as much as 30 g/day. Certain disease conditions cause rapid loss of plasma proteins; severe burns that denude large surface areas of the skin can cause the loss of several liters of plasma through the denuded areas each day.The rapid production of plasma proteins by the liver is valuable in preventing death in such states. Occasionally, a person with severe renal disease loses as much as 20 grams of plasma protein in the urine each day for months, and it is continually replaced mainly by liver production of the required proteins.
In cirrhosis of the liver, large amounts of fibrous tissue develop among the liver parenchymal cells, causing a reduction in their ability to synthesize plasma proteins.
Plasma Proteins as a Source of Amino Acids for the Tissues.
When the tissues become depleted of proteins, the plasma proteins can act as a source of rapid replace-ment. Indeed, whole plasma proteins can be imbibed in toto by tissue macrophages through the process of pinocytosis; once in these cells, they are split into amino acids that are transported back into the blood and used throughout the body to build cellular proteins wherever needed. In this way, the plasma proteins function as a labile protein storage medium and represent a readily available source of amino acids whenever a particular tissue requires them.
Reversible Equilibrium Between the Plasma Proteins and the Tissue Proteins. There is a constant state of equilibrium,as shown in Figure 69–2, among the plasma proteins, the amino acids of the plasma, and the tissue proteins. It has been estimated from radioactive tracer studies that normally about 400 grams of body protein are synthe-sized and degraded each day as part of the continual state of flux of amino acids. This demonstrates the general principle of reversible exchange of amino acids among the different proteins of the body. Even during starvation or severe debilitating diseases, the ratio of total tissue proteins to total plasma proteins in the body remains relatively constant at about 33:1.
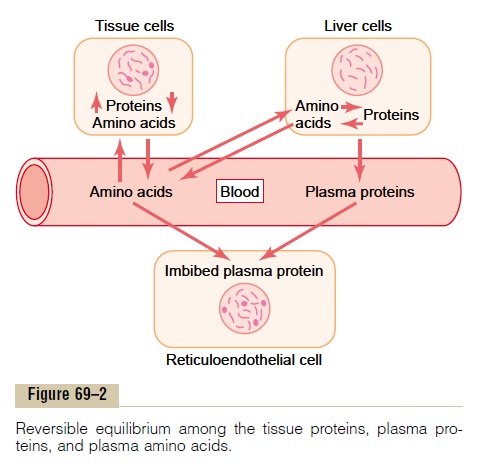
Because of this reversible equilibrium between plasma proteins and the other proteins of the body, one of the most effective therapies for severe, acute whole-body protein deficiency is intravenous transfusion of plasma protein. Within a few days, or sometimes within hours, the amino acids of the administered protein are distributed throughout the cells of the body to form new proteins where they are needed.
Essential and Nonessential Amino Acids
Ten of the amino acids normally present in animal pro-teins can be synthesized in the cells, whereas the other 10 either cannot be synthesized or are synthesized in quantities too small to supply the body’s needs. This second group of amino acids that cannot be synthesized is called the essential amino acids. Use of the word “essential” does not mean that the other 10 “nonessen-tial” amino acids are not required for the formation of proteins, but only that the others are not essential in thediet because they can be synthesized in the body.
Synthesis of the nonessential amino acids depends mainly on the formation of appropriate a-keto acids, which are the precursors of the respective amino acids. For instance, pyruvic acid, which is formed in large quantities during the glycolytic breakdown of glucose, is the keto acid precursor of the amino acid alanine. Then, by the process of transamination, an amino radical is transferred to the a-keto acid, and the keto oxygen is transferred to the donor of the amino radical. This reaction is shown in Figure 69–3. Note in this figure that the amino radical is transferred to the pyruvic acid from another chemical that is closely allied to the amino acids, glutamine. Glutamine is present in the tissues in large quantities, and one of its principal functions is to serve as an amino radical storehouse. In addition, amino radicals can be transferred from asparagine, glutamicacid, and aspartic acid.
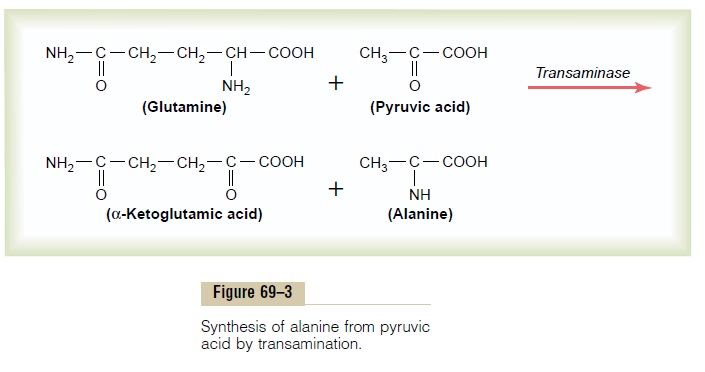
Transamination is promoted by several enzymes, among which are the aminotransferases, which are deriv-atives of pyridoxine, one of the B vitamins (B6). Without this vitamin, the amino acids are synthesized only poorly, and protein formation cannot proceed normally.
Use of Proteins for Energy
Once the cells are filled to their limits with stored protein, any additional amino acids in the body fluids are degraded and used for energy or are stored mainly as fat or secondarily as glycogen. This degradation occurs almost entirely in the liver, and it begins with deamina-tion, which is explained in the following section.
Deamination. Deamination means removal of the aminogroups from the amino acids. This occurs mainly by transamination, which means transfer of the amino group to some acceptor substance, which is the reverse of the transamination explained earlier in relation to the synthesis of amino acids.
The greatest amount of deamination occurs by the following transamination schema:
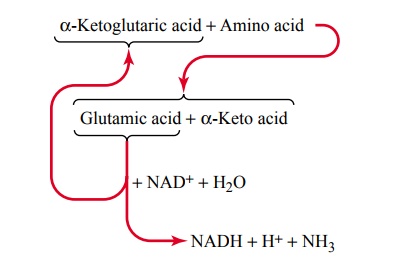
Note from this schema that the amino group from the amino acid is transferred to a-ketoglutaric acid, which then becomes glutamic acid. The glutamic acid can then transfer the amino group to still other substances or release it in the form of ammonia (NH3). In the process of losing the amino group, the glutamic acid once again becomes a-ketoglutaric acid, so that the cycle can be repeated again and again. To initiate this process, the excess amino acids in the cells, especially in the liver, induce the activation of large quantities of aminotrans-ferases, the enzymes responsible for initiating mostdeamination.
Urea Formation by the Liver. The ammonia releasedduring deamination of amino acids is removed from the blood almost entirely by conversion into urea; two mol-ecules of ammonia and one molecule of carbon dioxide combine in accordance with the following net reaction:
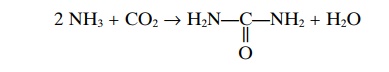
Essentially all urea formed in the human body is syn-thesized in the liver. In the absence of the liver or in serious liver disease, ammonia accumulates in the blood. This is extremely toxic, especially to the brain, often leading to a state called hepatic coma.
The stages in the formation of urea are essentially the following:
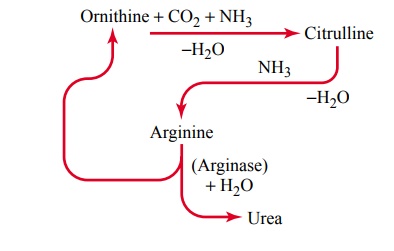
After its formation, the urea diffuses from the liver cells into the body fluids and is excreted by the kidneys.
Oxidation of Deaminated Amino Acids. Once amino acidshave been deaminated, the resulting keto acids can, in most instances, be oxidized to release energy for meta-bolic purposes. This usually involves two successive processes: (1) the keto acid is changed into an appro-priate chemical substance that can enter the citric acid cycle, and (2) this substance is degraded by the cycle and used for energy in the same manner that acetyl coen-zyme A (acetyl-CoA) derived from carbohydrate and lipid metabolism is used. In general, the amount of adenosine triphos-phate (ATP) formed for each gram of protein that is oxidized is slightly less than that formed for each gram of glucose oxidized.
Gluconeogenesis and Ketogenesis. Certain deaminatedamino acids are similar to the substrates normally used by the cells, mainly the liver cells, to synthesize glucose or fatty acids. For instance, deaminated alanine is pyruvic acid. This can be converted into either glucose or glycogen. Alternatively, it can be converted into acetyl-CoA, which can then be polymerized into fatty acids. Also, two molecules of acetyl-CoA can condense to form acetoacetic acid, which is one of the ketone bodies.
The conversion of amino acids into glucose or glyco-gen is called gluconeogenesis, and the conversion of amino acids into keto acids or fatty acids is called keto-genesis. Of the 20 deaminated amino acids, 18 havechemical structures that allow them to be converted into glucose, and 19 of them can be converted into fatty acids.
Obligatory Degradation of Proteins
When a person eats no proteins, a certain proportion of body proteins is degraded into amino acids and then deaminated and oxidized. This involves 20 to 30 grams of protein each day, which is called the obligatoryloss of proteins. Therefore, to prevent net loss ofprotein from the body, one must ingest a minimum of 20 to 30 grams of protein each day; to be on the safe side, a minimum of 60 to 75 grams is usually recommended.
The ratios of the different amino acids in the dietary protein must be about the same as the ratios in the body tissues if the entire dietary protein is to be fully usable to form new proteins in the tissues. If one particular type of essential amino acid is low in concentration, the others become unusable because cells synthesize either whole proteins or none at all in relation to protein synthesis. The unusable amino acids are deaminated and oxidized. A protein that has a ratio of amino acids different from that of the average body protein is called apartial protein or incompleteprotein, and such a protein is less valuable for nutritionthan is a complete protein.
Effect of Starvation on Protein Degradation. Except for the 20to 30 grams of obligatory protein degradation each day, the body uses almost entirely carbohydrates or fats for energy, as long as they are available. However, after several weeks of starvation, when the quantities of stored carbohydrates and fats begin to run out, the amino acids of the blood are rapidly deaminated and oxidized for energy. From this point on, the proteins of the tissues degrade rapidly—as much as 125 grams daily—and, as a result, cellular functions deteriorate precipitously. Because carbohydrate and fat utilization for energy normally occurs in preference to protein utilization, carbohydrates and fats are called proteinsparers.
Related Topics