Chapter: Medical Physiology: Transport of Oxygen and Carbon Dioxide in Blood and Tissue Fluids
Combination of Hemoglobin with Carbon Monoxide -Displacement of Oxygen
Combination of Hemoglobin with Carbon Monoxide -Displacement of Oxygen
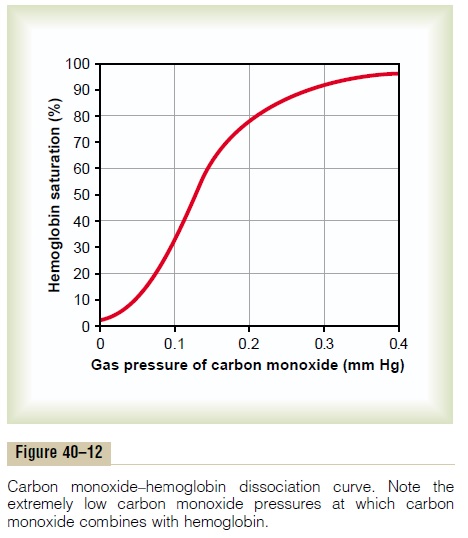
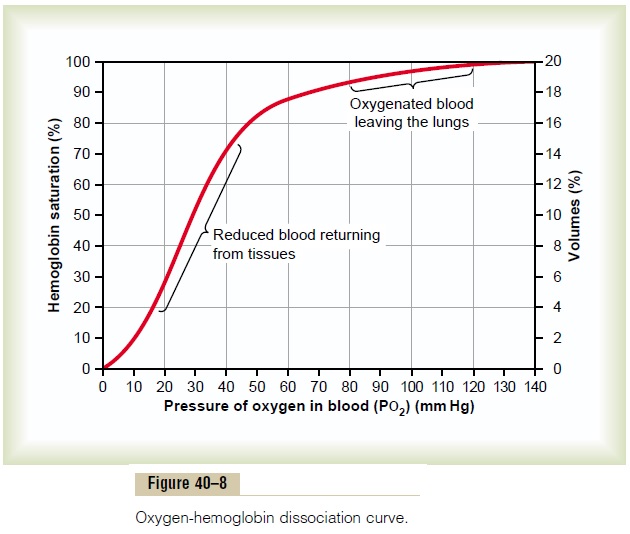
Carbon monoxide combines with hemoglobin at the same point on the hemoglobin molecule as does oxygen; it can therefore displace oxygen from the hemoglobin, thereby decreasing the oxygen carrying capacity of blood. Further, it binds with about 250 times as much tenacity as oxygen, which is demonstrated by the carbon monoxide–hemoglobin dissociation curve in Figure 40–12. This curve is almost identical to the oxygen-hemoglobin dissociation curve, except that the carbon monoxide partial pressures, shown on the abscissa, are at a level 1/250 of those for the oxygen-hemoglobin dis-sociation curve of Figure 40–8. Therefore, a carbon monoxide partial pressure of only 0.4 mm Hg in the alveoli, 1/250 that of normal alveolar oxygen (100 mm Hg PO2), allows the carbon monoxide to compete equally with the oxygen for combination with the hemoglobin and causes half the hemoglobin in the blood to become bound with carbon monoxide instead of with oxygen. Therefore, a carbon monoxide pressure of only 0.6 mm Hg (a volume concentration of less than one part per thousand in air) can be lethal.
Even though the oxygen content of blood is greatly reduced in carbon monoxide poisoning, the PO2 of the blood may be normal. This makes exposure to carbon monoxide especially dangerous, because the blood is bright red and there are no obvious signs of hypoxemia, such as a bluish color of the fingertips or lips (cyanosis). Also, PO2 is not reduced, and the feedback mechanism that usually stimulates increased respiration rate in response to lack of oxygen (usually reflected by a low PO2) is absent. Because the brain is one of the first organs affected by lack of oxygen, the person may become disoriented and unconscious before becoming aware of the danger.
A patient severely poisoned with carbon monoxide can be treated by administering pure oxygen, because oxygen at high alveolar pressure can displace carbon monoxide rapidly from its combination with hemoglo-bin. The patient can also benefit from simultaneous administration of 5 per cent carbon dioxide, because this strongly stimulates the respiratory center, which increases alveolar ventilation and reduces the alveolar carbon monoxide. With intensive oxygen and carbon dioxide therapy, carbon monoxide can be removed from the blood as much as 10 times as rapidly as without therapy.
Related Topics