Chapter: Modern Medical Toxicology: Chemical Poisons: Non-Metallic Chemical Poisons
Chlorine - Chemical Poisons
HALOGENS
Iodine
and iodides have been discussed under Caustics.
The other halogens of importance include chlorine,bromine, and fluorine. All
halogens combine with hydrogen to form acids, and with metals to form salts.
Physical Appearance
Chlorine
is a greenish-yellow gas with a pungent odour.
Uses/Sources
·
Chlorine is not found free in nature
due to its reactivity with other chemicals. Instead, it is found as sodium
chloride in land-locked lakes, as rock salt in underground deposits, in brines,
and in natural deposits of sylvite and carnallite
·
Swimming pool chlorinator tablets or
pellets may result in chlorine gas exposure.
·
Chlorine is used to manufacture a
number of chemicals including solvents such as carbon tetrachloride,
trichloroethylene, tetrachloroethylene, and methylene chloride, pesticides and
herbicides, plastics, vinyl chloride, etc. It is also used in making
refrigerants and propellants such as halocarbons and methyl chloride.
·
Chlorine is used to make sodium
hypochlorite, an ingredient in bleach, deodorisers and disinfectants. Household
bleach (5% sodium hypochlorite) when brought into contact with an acidic toilet
bowl cleaner or drain cleaner will cause the release of chlorine gas.
·
It is used extensively in pulpmills,
where wood chips are processed into pulp as part of the paper manufacturing
process.
·
Chlorine is employed in purifying
drinking and swimming water, for sanitation of industrial and sewage wastes and
other disinfecting uses.
·
It has been used as a poisonous gas
for military purposes under the name bertholite.
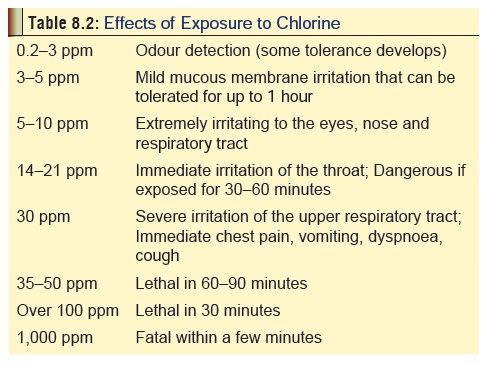
Usual Fatal Dose
Concentrations
of over 50 to 100 ppm when inhaled can be rapidly fatal. Instant death can
occur at concentrations over 1000 ppm. Table
8.2 gives an overview of effects to varying degrees of exposure to chlorine
gas.
Mode of Action
·
Chlorine is an extremely active
oxidising agent and causes rapid and extensive destruction of organic tissue.
It combines with tissue water to produce HCl, producing injury and reactive
oxygen species.
·
Chlorine gas in concentrated amounts
may be caustic to mucous membranes when inhaled or ingested; otherwise it is a
strong irritant. When in contact with moist tissue, nascent oxygen or “active
oxygen” is released as hydrogen is removed from H2O.
Nascent oxygen is a potent oxidiser, resulting in tissue damage. Secondary
irritation occurs from acids formed during this reaction.
·
Contact with respiratory epithelium
produces initial alve-olar capillary congestion followed by focal and confluent
patches of high fibrinogen oedematous fluid. Acute lung injury peaks in 12 to
24 hours. The fluid is interstitial at first but can fill the alveoli. Once
this occurs, copious frothy, blood-tinged sputum is observed.
Clinical Features
![]()
·
Chlorine is an irritant gas and
inhalation provokes rhinorrhoea, lacrimation, coughing, chest pain, and
shortness of breath.
·
Major exposure results in laryngeal
oedema, stridor, pneu-monitis, and pulmonary oedema.
·
In addition, the following features
of systemic toxicity are seen: vomiting, vertigo, headache, ventricular ectopic
beats, and metabolic acidosis.
·
Liquid chlorine can cause cutaneous
and mucosal burns.
·
Gaseous chlorine is a dermal
irritant and may cause burns in high concentrations
·
Chronic exposure to chlorine gas may
cause cough, sore throat, dyspnoea, palpitations, chest pain, reactive upper
airways dysfunction syndrome (RADS), dental enamel erosion, and an increased
susceptibility to viral respiratory infections.
o Conjunctivitis,
anosmia, and green discolouration of hair have also been reported.
o Chronic
exposure to chlorine gas is one of the most frequent causes of occupational
asthma.
Diagnosis
·
Characteristic odour.
·
Chlorine gas leak into the
atmosphere can be detected by opening a bottle of concentrated ammonium
hydroxide which will cause the production of heavy, white fumes of ammonium
chloride.*
Treatment
·
Mild poisoning can be managed with
bed rest and oxygen administration.
·
Cough can be controlled with codeine
and bronchodilators.
·
Nebulised sodium bicarbonate (3.75%
solution) is claimed to be effective in ameliorating respiratory symptoms by
neutralising the acid formed when chlorine comes into contact with water in the
airways. This can however provoke an exothermic reaction and doubts have been
expressed as to its efficacy and safety.
·
The role of corticosteroids in the
treatment of pulmonary oedema is also controversial.
·
Severe cases of poisoning will
require intermittent positive pressure ventilation.
·
Eye exposure must be treated with
copious irrigation of water or saline. Expert ophthalmic consultation is
advisable to rule out corneal damage.
Autopsy Features
·
Characteristic odour.
·
Massive pulmonary oedema.
·
Denudation of respiratory
epithelium.
Forensic Issues
Most
cases of poisoning are accidental arising out of domestic or industrial
exposure. Sometimes, exposure occurs at swim-ming pools where chlorine is often
used as a disinfectant.
Related Topics