Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Insulin
Chemical Description of Insulin
CHEMICAL DESCRIPTION
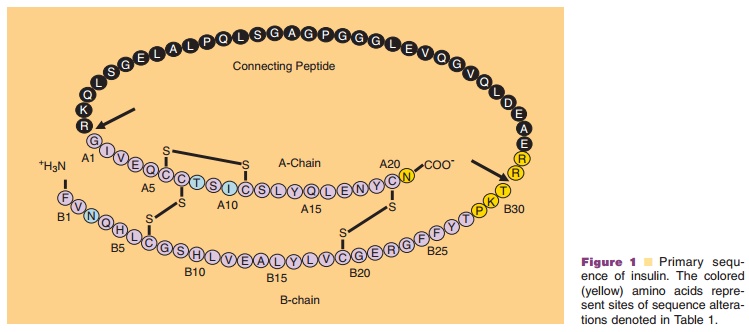
Insulin, a 51-amino acid protein, is a hormone that is synthesized as a
proinsulin precursor in the β-cell of the pancreas and is converted to insulin
by enzymatic cleavage. The resulting insulin molecule is composed of two
polypeptide chains that are connected by two interchain disulfide bonds (Fig.
1) (Baker et al., 1988). The A-chain is composed of 21 amino acids and the
B-chain is composed of 30 amino acids. The interchain disulfide linkages occur
between A7–B7 and A20–B19, respectively. A third intrachain disulfide bond is located in the
A-chain, between residues A6 and A11.
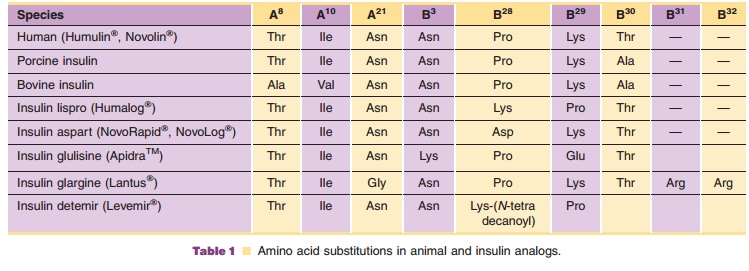
In addition to human insulin and insulin analog products, which are
predominately used today as the first-line therapies for the treatment of
diabetes, bovine and porcine insulin preparations have also been made
commercially available (Table 1; Fig. 1); however, all major manufacturers of
insulin have discontinued production of these products marking an end to future
supply of animal-sourced insulin products. Difficulties obtaining sufficient
supplies of bovine or porcine pancreata and recent concerns over transmissible
spongiform encephalopathies asso-ciated with the use of animal-derived
materials are major reasons for the product deletions.
The net charge on the insulin molecule is produced from the ionization
potential of four glutamic acid residues, four tyrosine residues, two histidine
residues, a lysine residue, and an arginine residue, in conjunction with two
α-carboxyl and two
α-amino groups. Insulin has an isoelectric point (pI) of5.3 in the denatured
state; thus, the insulin molecule is negatively charged at neutral pH
(Kaarsholm et al., 1990). This net negative charge-state of insulin has been
used in formulation development, as will be discussed later.
In addition to the net charge on insulin, another important intrinsic
property of the molecule is its ability to readily associate into dimers and
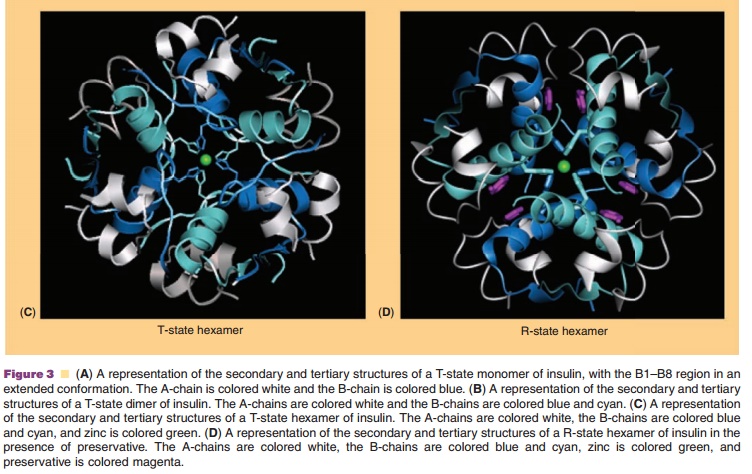
higher order states (Figs. 2 and 3) (Pekar and Frank, 1972). The driving force
for dimerization appears to be the formation of favorable hydrophobic
interactions at the C-terminus of the B-chain (Ciszak et al., 1995). Insulin
can associate into discrete hexameric complexes in the presence of various
divalent metal ions, such as zinc at 0.33 g-atom/monomer (Goldman and
Carpenter, 1974), where each zinc ion (a total of two) is coordinated by a HisB10 residue from three mono-mers.
Physiologically, insulin is stored as a zinc-containing hexamer in the β-cells
of the pancreas. As will be discussed later, the ability to form discrete
hexamers in the presence of zinc has been used to develop therapeutically
useful formulations of insulin.
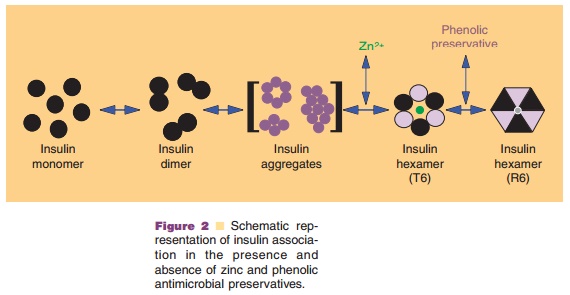
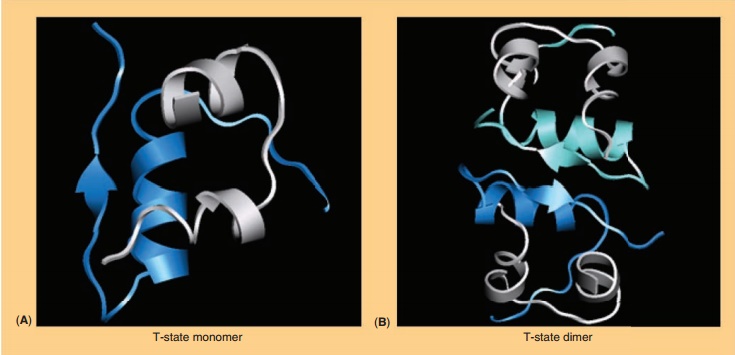
Commercial insulin preparations also contain phenolic excipients (e.g., phenol, m-cresol, or methyl-paraben) as antimicrobial agents. As represented in Figures 2 and 3D, these phenolic species also bind to specific sites on insulin hexamers, causing a con-formational change that increases the chemical stabi-lity of insulin in commercial preparations (Brange and Langkjær, 1992). X-ray crystallographic data have identified the location of six phenolic ligand-binding sites on the insulin hexamer and the nature of the conformational change induced by the binding of these ligands (Derewenda et al., 1989). The phenolic ligands are stabilized in a binding pocket between monomers of adjacent dimers by hydrogen bonds with the carbonyl oxygen of CysA6 and the amide proton of CysA11 as well as numerous van der Waals contacts. The binding of these ligands stabilizes a conformational change that occurs at the N-terminus of the B-chain in each insulin monomer, shifting the conformational equilibrium of residues B1 to B8 from an extended structure (T-state) to an α-helical struc-ture (R-state). This conformational change is referred to as the T<–> R transition (Brader and Dunn, 1991) and is illustrated in Figure 3D.
In addition to the presence of zinc and phenolic preservatives, modern
insulin formulations may con-tain an isotonicity agent (glycerol or NaCl)
and/or a physiologic buffer (sodium phosphate). The former is used to minimize
subcutaneous tissue damage and pain on injection. The latter is present to
minimize pH drift in some pH-sensitive formulations.
Related Topics