Chapter: Biotechnology: Animal Cell Culture and Applications
Animal Cell Culture Techniques
Animal Cell Culture Techniques
Features of animal cell growth in culture
Animal cells can be grown in glass or plastic vessels, in the presence of nutritive media that need to be is periodically replenished. However, depending on the tissue they have been isolated from, they can be grown only for limited generations even in the best nutritive media. There is a mortality associated with all normal animal cells. Another important feature of animal cells is that they divide and fill the surface of the culture vessel and then stop growing. Relate this to what happens in the animal body. The infant animal grows only to adulthood and not any further. Cells comprising tissues and organs such as the liver grow only to a certain size after which they cease to grow. This phenomenon which occurs in the normal body is observed also in cell culture and is termed "contact inhibition". This means that when cells grow and reach the walls of the container (i.e., reach confluency) they stop growing further. Another important feature of cell growth in culture is that their environment is different from that in vivo. These differences affect the adherence of cells to culture vessels, their shape and rate of proliferation. It is of interest to know that in culture, cancer cells appear very different from normal cells. Cancer cells loose contact inhibition and pile on each other due to uncontrolled growth and among other features, appear more rounded in shape. Such differences in growth patterns in normal versus cancer cells are utilized by Oncologists (cancer biologists) to determine whether tumors are cancerous or not using `Colony formation assay'. Let us now learn about various types of cell cultures and the technology associated with it.
Primary Cell Cultures
Cells are dissociated from the parental tissue (such as kidney, liver) by mechanical or enzymatic methods and maintained in suitable culture medium and vessels. The most frequently used enzymes for separating cells from a given tissue (dispersion) are crude preparations of trypsin and collagenase that cleave the proteinaceous cementing material between cells in a tissue. The maintenance of growth of such cells under laboratory conditions is known as primary cell culture.
The characteristics of cells in culture usually depend on their original source within the animal.
Cells can be grown as adherent (anchorage-dependent) or suspension cultures (anchorage-independent). Adherent cells are usually derived from tissues of organs such as kidney where they are not mobile and are embedded in connective tissue. They grow adhering to the cell culture vessel. On the other hand, suspension cells do not attach to the surface of the culture vessel. Virtually all suspension cultures are derived from cells of the blood system. This is because, these cells (e.g., lymphocytes) are also suspended in plasma in vivo. The drawbacks of primary culture are that they are time consuming and require the use of live animals or fresh tissue. There can be considerable variation from one preparation to another particularly if prepared by different people. These difficulties can be overcome by the use of secondary cell cultures or cell lines.
Secondary Cell Cultures and Cell Lines
Once the primary culture is subcultured, it is known as secondary culture or cell line. Subculturing or "splitting cells," is required to periodically provide fresh nutrients and growing space for continuously growing cell lines. The frequency of subculture or density of cells to be plated, depends on the characteristics of each cell type. If cells are split too frequently or at too low a density, the line may be lost. If cells are not split frequently enough, the cells may exhaust the medium and die. Sub-culturing involves: removing the growth media, washing the plate, disassociating the adhered cells, usually enzymatically (e.g. with trypsin), although some cells may be removed by repeated pipeting or gentle scraping), and diluting the cell suspension into fresh media. Rous and Jones were first to introduce proteolytic enzyme trypsin for the subculture of adherent cells. Such cultures may be called secondary cultures. Sometimes, certain cells of these secondary cell cultures can spontaneously become altered (transformed) and give rise to continuous cell lines which show immortality, as they can grow indefinitely without dying in culture. These cultures can contain mixed cell types or can consist predominantly of a single cell type.
Types of Cell Lines
The various types of cell lines are categorized into two types, i.e., finite cell line and continuous cell line.
Finite Cell Lines
Finite cell lines are those cell lines which have a limited life span and they grow through a limited number of cell generations. Finite cell lines show the property of contact inhibition, density limitation and anchorage dependence. The mode of growth is in the monolayer form. The growth rate is slow and doubling time is around 24 to 96 hours.
Continuous Cell Lines
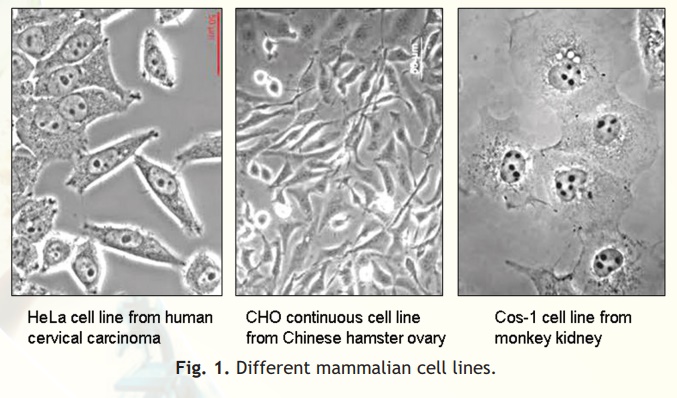
Cell lines transformed under in vitro culture conditions give rise to continuous cell lines (Fig. 1). The various properties associated with continuous cell lines are: theploidy (change in basic number of chromosomes), no contact inhibition and no anchorage dependence. The mode of growth is either monolayer or suspension form. The growth rate is rapid and doubling time is between 12 to 24 hours. The density limitation is reduced or lost.
Physical environment for culturing Animal Cells
The culturing of animal cells under in vitro condition involves creation of appropriate physical, nutritional and hormonal environments in which the cells can grow. The physical environment includes controlling the temperature, pH, osmolality and gaseous environment by providing a supporting surface and protecting the cells from chemical, physical and mechanical stresses.
Temperature
The mammalian cells are grown in incubators maintained at 37°C. This temperature is chosen because it is the core body temperature of Homo sapiens. Further, it has been observed that most cells derived from the warm blooded animals will grow at this temperature.
pH
The regulation of extra-cellular and intra-cellular pH is essential for the survival of mammalian cells. The correct pH is not only important for maintaining the appropriate ion balance but also for maintaining optimal function of cellular enzymes and for optimal binding of hormones and growth factors to cell surface receptors. Even transient changes in pH can alter cell metabolism which can lead to cell death. Most media strive to achieve and maintain the pH between 7 and 7.4. The regulation of pH is done using a variety of buffering systems. Most media use the bicarbonate - CO2 buffering system. The interaction of CO2 derived from cells or atmosphere with water leads to a drop in pH described by the equation below:

Osmolality
The osmolality of the culture medium also has a significant bearing on cell growth and function. It preserves the membrane integrity of cells. If the outside osmotic pressure becomes higher or lower than that which must be maintained inside the cell, it will shrink or swell accordingly. The osmolality of the medium used is determined by the media formulation. Salt and glucose are the major contributors to the osmolality of the medium, although amino acids may also contribute significantly. Almost all commercial media are formulated to have a final osmolality of around 300 mOsm. Osmolality can be checked directly with an osmometer.
Medium
The most commonly varied factor in culture systems is the growth medium. Medium is a mixture of inorganic salts and other nutrients capable of sustaining cell survival in vitro. Having the correct nutrient mixture can often be the determining factor in failure or success in cell culture. The medium provides essential nutrients that are incorporated into dividing cells, such as, amino acids, fatty acids, sugars, ions, trace elements, vitamins, cofactors, and ions necessary to maintain the proper chemical environment for the cell. Some components may perform both roles; for example, the sodium bicarbonate may be used as a carbonate source but also may play an important role in maintaining the appropriate pH and osmolality. All media contain an energy source, usually glucose. Many of the media contain phenol red as a pH indicator. This is very helpful in monitoring the pH of the culture medium in an incubator. Highly acidic conditions turn the phenol red into yellow while highly alkaline conditions turns the phenol red into pink color.
Serum and antibiotics
Serum is one of the most important components of animal cell culture, as it supports cell proliferation and their attachment to culture vessels. The peptide hormones or hormone-like growth factors that promote healthy growth are often derived from animal blood, such as foetal bovine serum (FBS). Serum is also a source of various amino acids, hormones, lipids, vitamins, polyamines and salts containing ions such as calcium, chloride, ferrous, ferric, potassium etc. Current practice is to minimize using blood-based supplements and switch to serum-free medium due to some complications of FBS usage. Although not required for cell growth, antibiotics such as penicillin and streptomycin are often used in culture medium to control the growth of bacterial and fungal contaminants.
Vessels and Equipments required for Animal Cell Culture
Cultures should be examined daily for their morphology, colour of the medium and density of cells. The animal cells are usually grown and maintained in Petri dishes, Culture flasks or Multi-well plates of various shapes and sizes (Fig. 2) at an appropriate temperature and gas mixture (typically, 37°C, 5% CO2 for mammalian cells) in an incubator. Culture conditions vary widely for each cell type, and variation of conditions for a particular cell type can result in different phenotypes being expressed.
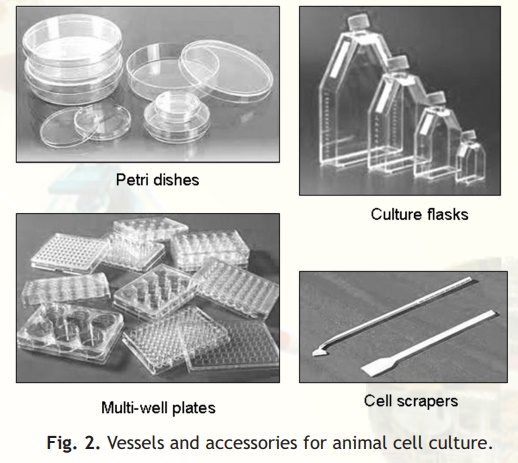
Fig. 2. Vessels and accessories for animal cell culture.
A cell culture laboratory should have equipments like tissue culture hood, CO2 incubator, inverted microscope, Centrifuge etc. for doing animal cell culture work.
Tissue Culture Hood
All tissue/cell culture manipulations must be performed asceptically, i.e., without any bacterial or fungal contamination. Otherwise, animal cell culture media can easily get contaminated with bacteria or fungi which will outgrow animal cells. The Laminar Air Flow (LAF) hoods allow the work area to be free of such contamination. A LAF hood essentially performs two functions:
1. Protects the tissue culture from the operator (by providing a sterile environment).
2. Protects the operator from the tissue culture (from possible infection risk).
Depending on the nature of cells/tissue being handled (especially infective agents), the biology safety cabinets are designated as Class I to class III. The LAF hoods have continuous displacement of air that passes through a high efficiency particle air (HEPA) filter that removes particulates from the air. The hoods are equipped with a short-wave UV light source that can be turned on for a few minutes to sterilize the surfaces of the hood just before use.
CO2 Incubator
The CO2 incubator is designed to reproduce as closely as possible the environmental conditions of the living cells. The essential functions of the incubator are to maintain, the sterility of the chamber, a constant temperature, an atmosphere with a fixed level of CO2 and high relative humidity. A pan of water is kept at all times in the incubator chamber to maintain high relative humidity and prevent desiccation of the culture medium and maintain the correct osmolarity (Fig. 3A). The animal cells are grown in an atmosphere of 5-10% CO2 because the medium used is buffered with sodium bicarbonate/carbonic acid and the pH must be strictly maintained.
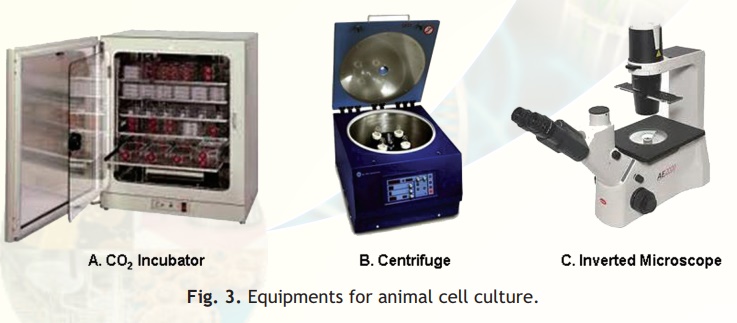
Fig. 3. Equipments for animal cell culture.
Centrifuge
For most cell culture only low-speed centrifuges are required (Fig. 3B). A gentle braking action helps prevent disruption of the separated bands of cells. In most cases cells should be centrifuged at 20°C; nevertheless low operation temperature is useful to avoid exposing cells to uncontrolled higher temperatures.
Inverted Microscope
In tissue culture vessels, for example a petri dish, the cells are present at the bottom with the culture medium above. The inverted microscope allows the cells at the bottom to be visualized because the optical system is at the bottom with the light source on top (Fig. 3C). Observation of cultures in this way will give an immediate idea of the health and growth of cells. Microscopes should be kept covered to protect from dust and the lights turned down when not in use.
Related Topics