Chapter: Modern Pharmacology with Clinical Applications: Adrenomimetic Drugs
Adrenomimetic Drugs: Mechanism of Action
MECHANISM OF
ACTION
Many adrenomimetic drugs produce responses by inter-acting with the adrenoceptors on sympathetic effector cells. An examination of Table 9.1 reveals that sympa-thetic effectors have activity at α1-, α 2-,β 1-, or β 2-adrenoreceptors or in some cases, combinations of these adrenoceptors. Adrenomimetic drugs vary in their affini-ties for each subgroup of adrenoceptors. Some, like epi-nephrine, have a high affinity for all of the adrenoceptors.
Others are relatively
selective. For example, isopro-terenol has a high affinity for β 1- and β 2-adrenoceptors but a very low
affinity for α -adrenoceptors; isopro-terenol is considered a nearly pure β -agonist. Norepinephrine has
a high affinity for α- and β 1-adrenoceptors but a relatively low affinity for β 2-receptors.
The effect of a given adrenomimetic drug on a partic-ular type of
effector cell depends on the receptor selectiv-ity of the drug, the response
characteristics of the effector cells, and the predominant type of adrenoceptor
found on the cells. For example, the smooth muscle cells of many blood vessels have only or predominantly α-adrenocep-tors. The
interaction of compounds with these adreno-ceptors initiates a chain of events
in the vascular smooth muscle cells that leads to activation of the contractile
process. Thus, norepinephrine and epinephrine, which have high affinities for α-adrenoceptors, cause the
vas-cular muscle to contract and the blood vessels to con-strict. Since
bronchial smooth muscle contains β 2-adrenoceptors, the response in this tissue elicited
by the action of β 2-adrenoceptor agonists is relaxation of smooth muscle cells.
Epinephrine and isoproterenol, which have high affinities for β 2-adrenoceptors, cause
re-laxation of bronchial smooth muscle. Norepinephrine has a lower affinity for
β 2-adrenoceptors and has
rela-tively weak bronchiolar relaxing properties.
Adrenomimetic drugs can be
divided into two major groups on the basis of their mechanism of action.
Norepinephrine, epinephrine, and some closely related adrenomimetics produce
responses in effector cells by directly stimulating α- or β-adrenoceptors and are
re-ferred to as directly acting
adrenomimetic drugs.
Many other adrenomimetic
drugs, such as ampheta-mine, do not themselves interact with adrenoceptors, yet
they produce sympathetic effects by releasing norepi-nephrine from neuronal
storage sites (vesicles). The norepinephrine that is released by these
compounds interacts with the receptors on the effector cells. These
adrenomimetics are called indirectly
acting adreno-mimetic drugs. The
effects elicited by indirectly acting drugs
resemble those produced by norepinephrine.
An important characteristic
of indirectly acting adrenomimetic drugs is that repeated injections or
pro-longed infusion can lead to tachyphylaxis
(gradually di-minished responses to repeated administration). This is a result
of a gradually diminishing availability of re-leasable norepinephrine stores on
repeated drug ad-ministration. The time frame of the tachyphylaxis will vary
with individual agents.
The actions of many
indirectly acting adreno-mimetic drugs are reduced or abolished by the prior
ad-ministration of either cocaine or tricyclic antidepressant drugs (e.g.,
imipramine). These compounds can block the adrenergic neuronal transport system
and thereby prevent the indirectly acting drug from being taken up into the
nerve and reaching the norepinephrine storage vesicles. Lipophilic drugs (e.g.,
amphetamine), however, can enter nerves by diffusion and do not need mem-brane
transport systems.
Destruction or surgical
interruption of the adrener-gic nerves leading to an effector tissue renders
indirectly acting adrenomimetic drugs ineffective because neu-ronal
norepinephrine is no longer available for release since the nerves have
degenerated. Also, patients being treated for hypertension with reserpine or
guanethidine, which deplete the norepinephrine stores in adrenergic neurons ,
respond poorly to administra-tion of indirectly acting adrenomimetic drugs.
Some adrenomimetic drugs act
both directly and in-directly; that is, they release some norepinephrine from
storage sites and also directly activate tissue receptors. Such drugs are
called mixed-action adrenomimetics.
However, most therapeutically important adreno-mimetic drugs in humans act
either directly or indirectly.
Structure–Activity Relationships Among Adrenomimetic Drugs
The nature of the
substitutions made on the basic phenylethylamine skeleton at the para and meta
positions of the benzene ring or on the -carbon of the side chain determine
whether an adrenomimetic drug will act di-rectly or indirectly. Directly acting
adrenomimetic drugs, which have two or more carbon atoms (e.g., isopro-terenol)
added to their amino group, are virtually pure β-adrenoceptor agonists. Directly acting drugs,
which have only small substitutions on their amino groups (e.g., nor-epinephrine
and epinephrine), are usually β-adrenocep-tor agonists, but may be α-adrenoceptor agonists as well. Norepinephrine
has very weak actions on β2-adrenocep-tors but strong β1-adrenoceptor actions. Epinephrine has a high
affinity for both β1- and β2-adrenoceptors.
Adrenomimetic drugs with no
substitutions on their benzene ring (e.g., amphetamine and ephedrine) are
generally quite lipid soluble, readily cross the blood-brain barrier, and can
cause central nervous system (CNS) stimulation.
The structure of a particular
adrenomimetic drug will influence its susceptibility to metabolism by catechol-O-methyltransferase (COMT) and monoamine
oxidase (MAO). The actions of COMT
are specific for the cate-chol structure. If either the meta or para hydroxyl
group is absent, COMT will not metabolize the drug. The pres-ence of a
substitution, such as a methyl group, on the - carbon of the side chain reduces
the affinity of the adrenomimetic drug for MAO. Also, drugs with a large
substitution on the terminal nitrogen will not be de-graded by MAO. A
noncatecholamine that has a methyl group attached to its -carbon will not be
metabolized by either enzyme and will have a greatly prolonged du-ration of
action (e.g., amphetamine).
The Role of Second Messengers in Receptor-mediated Responses
The adrenomimetic drugs,
including the naturally oc-curring catecholamines, initiate their responses by
com-bining with α-, β-, or dopamine adrenoceptors. This in-teraction triggers a series
of biochemical events starting within the effector cell membrane that
eventually cul-minates in the production of a physiological response, for
example contraction, secretion, relaxation, or al-tered metabolism. The total
process of converting the action of an external signal (e.g., norepinephrine
inter-acting with its receptor) to a physiological response (e.g., vascular
smooth muscle contraction) is called sig-nal
transduction.
Following the binding of the
agonist (the first mes-senger) to its
appropriate receptor on the external sur-face of the effector cell, a second messenger is generated (or
synthesized) and participates in a particular series of biochemical reactions
that ultimately result in the gen-eration of a specific physiological response
by that cell (Figs. 10.2 and 10.3). For both α- and β-adrenoceptors, the signal transduction process
seems to involve the participation of G proteins .
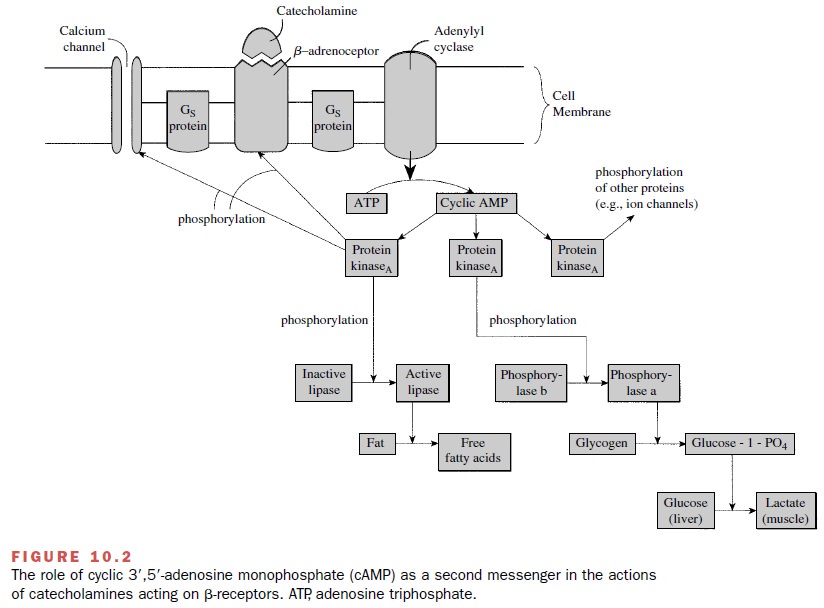
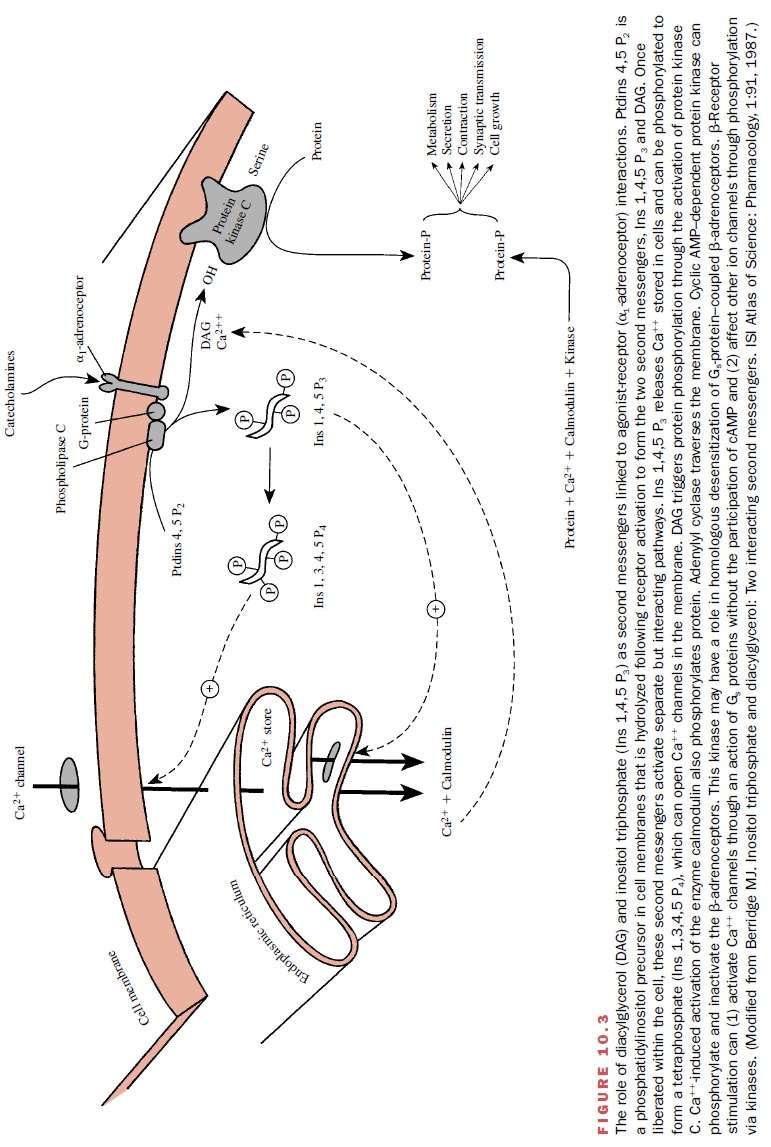
The specific second-messenger pathways constitute a highly versatile signaling system that can modify (stimu-late or inhibit) numerous cellular processes including secretion, contraction and relaxation, metabolism, neu-ronal excitability, cell growth, and apoptosis. The sec-ond messengers that participate in signal transduction include cyclic adenosine monophosphate (cAMP), diacylglycerol, and inositol triphosphate. Once liberated within the cell, second messengers will activate specific signal pathways.
For example,
inositol triphosphate functions by mobilizing calcium from intracellular stores
or opening channels; the calcium can be used to initi-ate vascular smooth
muscle contraction, probably through a protein phosphorylation pathway (Fig.
10.3). Diacylglycerol is known to stimulate an enzyme, protein kinase C, that
phosphorylates specific intracellular pro-teins, some of which regulate ionic
mechanisms such as the NA+ /H+ exchanger and potassium
channels.
The basic features of the
signaling system found in different cells are remarkably similar. It appears
that protein phosphorylation is a final common pathway in the molecular mechanisms
through which neurotrans-mitters, hormones, and the nerve impulse produce many
of their biological effects in target cells.
Related Topics