Chapter: Clinical Cases in Anesthesia : Intraoperative Coagulopathies
What are the most common intraoperative coagulopathies?
What are the most common intraoperative coagulopathies?
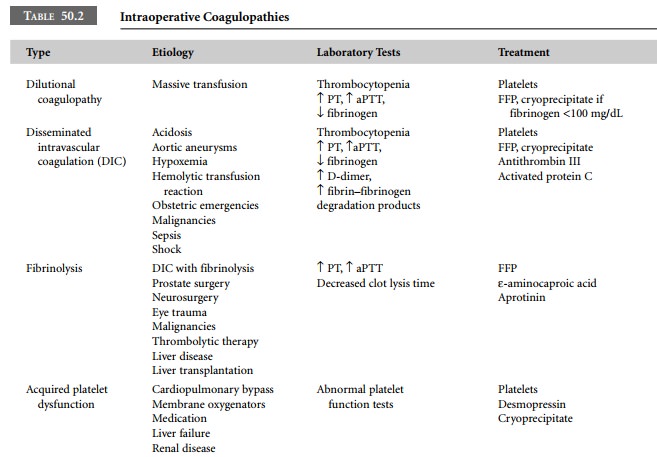
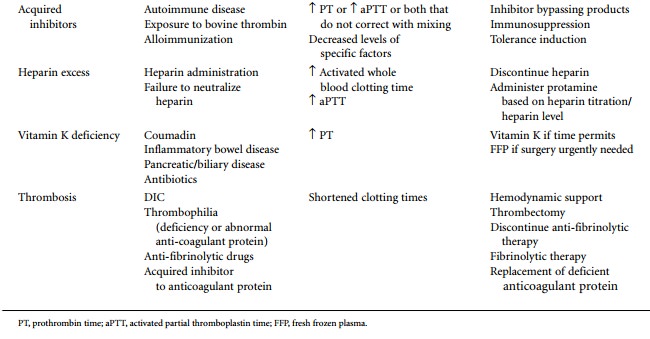
The common intraoperative coagulopathies are
listed in Table 50.2. They include dilutional coagulopathies secondary to
massive transfusion, disseminated intravascular coagulation (DIC),
fibrinolysis, acquired platelet dysfunc-tion, acquired inhibitors, heparin
excess, vitamin K defi-ciency, and thrombosis.
Dilutional coagulopathy secondary to massive
transfu-sion is the most common intraoperative coagulopathy. Massive
transfusion may be defined as the transfusion of greater than 10 units of
blood, replacement of 50% of the circulating blood volume in 3 hours, or
replacement of one blood volume in 24 hours. During surgery, maintenance of
blood volume by appropriate administration of crystal-loids and colloids is
essential. Red blood cells (RBCs) are transfused as needed to provide oxygen
carrying capacity. Whole blood is virtually unavailable because all donor units
collected are used for component preparation. As a consequence, packed RBCs or
RBCs in adenine/saline pre-servative solution are the primary products used.
RBC products contain no viable platelets. Most platelets are removed from the
unit during the preparation of platelet concentrate; the rest are inactivated
after storage at 1–6°C for 24 hours. At a minimum, 48–72 hours is
needed to complete the required infectious disease testing. Thus, platelets in
RBC products and platelets in whole blood that may have been collected for a
special procedure are invari-ably inactivated. RBC products also contain little
plasma. Conventional packed RBCs contain a small amount of residual plasma.
However, most RBCs are prepared in adenine/saline preservative solution to
extend the shelf life to 42 days. After centrifugation of a unit of whole
blood, as much plasma as possible is removed and replaced with 100–110 mL of
the preservative solution.
The
hematocrit of adenine/saline RBCs is 55–65%, whereas the hematocrit of
conventional packed cells is 70–80%. The transfusion of crystalloid and/or
colloid solutions with RBCs creates a dilutional coagulopathy manifested by
thrombocytopenia and prolongation of coagulation tests. Abnormal bleeding does
not invariably occur when these laboratory abnormalities are present. During
massive transfusion, hemostasis should be rou-tinely monitored with
point-of-care testing. When exces-sive bleeding occurs, the results of
coagulation tests should be used to guide replacement therapy. Excessive
microvas-cular bleeding appears to be more common with platelet counts less
than 50,000/μL and fibrinogen levels below mg/dL.
DIC is a syndrome characterized by primary
intravas-cular activation of the coagulation system with secondary activation
of fibrinolytic pathways. Although the mecha-nism of activation may vary,
proinflammatory cytokines (interleukin-6, interleukin-1β, and tumor necrosis factor-α) and thrombin generation through the tissue
factor/VIIa extrinsic factor pathway are major factors in the develop-ment of
DIC. Activation of the coagulation cascade leads to the formation of
intravascular thrombi and consumption of platelets, clotting factors, and
anticoagulant proteins (protein C, protein S, antithrombin III).
Although there is secondary activation of the fibrinolytic
pathway, there may be impaired fibrin degradation because circulating levels of
plasminogen activator inhibitor-type 1 are increased. In the intraoperative
setting, bleeding may be the presenting symptom because of consumption of
platelets and coagulation proteins. However, occlusion of small and
medium-sized vessels may cause multisystem organ dys-function and failure.
Laboratory studies demonstrate a prolonged PT, aPTT, decreased fibrinogen
levels, increased D-dimer, and increased fibrin-fibrinogen degradation
products. Antithrombin III and protein C levels are also decreased.
DIC may complicate many clinical conditions. It
may be clinically silent and compensated or it may be uncompen-sated and
flagrant. DIC occurs in sepsis, aortic aneurysms, massive trauma, placental
abruption, eclampsia, amniotic fluid embolism, fetal death in utero, and with
brain injuries. Patients with solid tumors and hematologic malignancies may
also have DIC. In the patient with normal hemostasis prior to surgery, shock,
acidosis, hypoxia, or endotoxemia may trigger DIC. The entry of brain tissue,
fat or tumor into the circulation may also activate coagulation.
The treatment of DIC is controversial,
particularly in the surgical setting. There is general agreement that control
of the underlying disease is essential. When hemorrhagic symptoms predominate,
transfusion of platelets, plasma, and cryoprecipitate is indicated. Since
levels of anticoagu-lant proteins are also low, the use of antithrombin III
(ATIII) and activated protein C should be considered. ATIII concentrates have
been used in congenital antithrombin deficiency, in obstetric emergencies, to
treat heparin resistance, and in DIC associated with sepsis. The results in
sepsis have been variable but many obstetricians believe that ATIII is
invaluable in certain clinical settings. Unfortunately, ATIII concentrate has
been in very short supply recently because of decreased production and FFP is
the only alternative product.
Recombinant activated protein C was recently
licensed in the United States to treat severe sepsis. In clinical trials it
decreased levels of D-dimer and interleukin-6 and reduced mortality. Activated
protein C has also been used to man-age DIC in obstetric patients in Japan.
Post-marketing studies may identify other clinical conditions with DIC in which
use of this product decreases morbidity and reduces mortality. Clinical trials
are also under way to evaluate tissue factor pathway inhibitor in sepsis.
Fibrinolysis occurs when massive activation of
plas-minogen overwhelms the fibrinolytic inhibitor system. Urologic surgery,
pulmonary and cardiovascular surgery, brain trauma, and eye injury may cause
such activation. Epsilon-aminocaproic acid and aprotinin are effective antifibrinolytic
drugs in these circumstances. Fibrinolysis may also be iatrogenic resulting
from thrombolytic therapy. Secondary fibrinolysis in DIC may contribute to
intraoperative bleeding.
Patients with severe liver disease who need
surgery may also have problems with fibrinolysis. Thrombocytopenia and multiple
coagulation deficiencies are present in severe liver disease. Patients often
have dysfibrinogenemias and decreased hepatic clearance of fibrin degradation
products causing poor clot formation. Delayed clearance of plas-minogen
activator and reduced hepatic synthesis of inhibitor proteins such as α2-antiplasmin and histidine-rich glycoprotein increase fibrinolytic
activity.
The coagulopathy of liver transplantation is
also associ-ated with massive fibrinolysis. Shortly after the donor liver is
reperfused, massive fibrinolysis is caused by release of tissue-type
plasminogen activator from the donor liver. Both ε-aminocaproic acid and aprotinin have been used
in this setting.
Acquired platelet dysfunction is relatively
common. Usually it is caused by medications that inhibit platelet func-tion and
that are not discontinued prior to surgery. Many of these drugs are medications
used in cardiovascular disease. Abciximab, tirofiban, epitifibatide, and clopidogrel
are prominent because of the frequency with which they are used. Also, because
of their frequent exposure to heparin, patients with cardiovascular disease may
also have heparin-induced thrombocytopenia and associated platelet
dys-function. Many other medications can cause platelet dysfunction. Volume
expanders such as dextran and hydroxy-ethyl starch and high doses of penicillin
and cephalosporins may contribute to surgical bleeding. Finally, in cardiac
sur-gery, bypass produces defective platelet function, probably related to
platelet activation and fragmentation.
Acquired inhibitors are infrequent but dramatic
causes of intraoperative coagulopathy. Most inhibitors occur in severe
hemophilia where antibody develops following exposure to factor VIII or IX when
concentrate is used for prophylaxis or therapy. These patients are invariably
identified in the pre-operative screening process and appropriate plans for
treat-ment and monitoring planned well in advance. The development of
recombinant activated factor VIIa to treat such patients has reduced mortality
and morbidity in this group of patients. Acquired inhibitors may develop in
indi-viduals with no history of abnormal bleeding. These inhibitors are
autoantibodies to autologous coagulation pro-teins. The most commonly reported
acquired inhibitors are to factor VIII, although inhibitors to other clotting
factors have been reported. These inhibitors may occur in preg-nancy,
postpartum, in autoimmune diseases such as lupus, with solid tumors, hematologic
malignancies, and in the eld-erly. Recently there has been an alarming increase
in reports of acquired inhibitors to factor V in patients exposed to bovine
thrombin preparations when “homemade” fibrin sealants were made from
cryoprecipitate. Most of these patients have had neurosurgery or cardiovascular
surgery, but fibrin sealant is useful in many procedures. The com-mercially
available fibrin sealant (Tisseel) does not use bovine thrombin and is an
alternative and preferred product.
Screening coagulation tests are abnormal with
acquired inhibitors. However, when a patient urgently requires surgery and
there is no history of abnormal bleeding, they are sometimes taken to surgery
under the assumption that FFP will correct the abnormality.
If the inhibitor is identified prior to surgery
the proce-dure should be postponed if possible. Some inhibitors spontaneously
disappear, while others respond to immunosuppressive treatment. If surgery is
urgently needed, plasma exchange or immunoadsorption to reduce the titer of the
inhibitor in combination with replacement therapy and/or factor bypassing
products such as rVIIa and FEIBA may be helpful.
Related Topics