Test, Equipments, Procedure, Result - Urine Analysis | 11th Nursing : Chapter 8 : Nursing Procedures
Chapter: 11th Nursing : Chapter 8 : Nursing Procedures
Urine Analysis
Urine Analysis
Test for sugar - benedict’s test
Benedict’s test is
used as a simple test for reducing sugars. A reducing sugar is a carbohydrate
possessing either a free aldehyde or free ketone functional group as part of
its molecular structure. This includes all monosaccharides (eg. glucose,
fructose, galactose) and many disaccharides, including lactose and maltose.
Benedict’s test is
most commonly used to test for the presence of glucose in urine. Glucose found
to be present in urine is an indication of Diabetes mellitus
Equipments
Benedict’s solution
(fresh; certainly not more than 3 months old), Dropper, Test-tube, Test-tube
holder. Spirit lamp, Match box, Kidney tray, containers.
Quality checking of the Benedict’s solution
Benedict’s solution is
blue in color. In order to check purity of Benedict’s solution take 5 ml of
Benedict’s solution in test tube and heat it. If it does not change color, it
means, it is pure. Spirit lamp, Match box, Kidney tray, Container.
Procedure
·
Take 5 ml (one teaspoon) of Benedict’s solution in the
test-tube.
·
Holding the test-tube with the holder, heat it over a spirit
lamp till the Benedict’s Solution boils without overflowing.
·
Drop 8 to 10 drops of urine into the boiling Benedict’s
solution.
·
After again boiling the mixture, let it cool down.
·
While cooling, the mixture changes color.
·
Observe the color change and precipitate formation and analyze
the test result
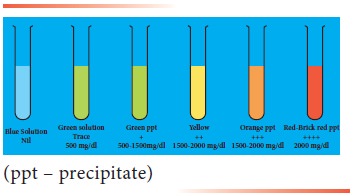
Result interpretation
The colour of the
mixture serves as a guide to the amount of sugar in the urine :
Test For Albumin
·
Fill three-fourth of a test tube with filtered urine (filtering
removes pus if present).
·
See the reaction of the urine is acidic. If found alkaline, add
one drop of acetic acid and make it acidic.
·
Heat the upper third of the urine over sprit lamp and allow it
to boil.
·
A cloud may appear either due to phosphate or albumin.
·
Add acidic acid drop by drop in to the test tube.
·
If the urine is still cloudy it indicates the presence of
albumin.
·
If it becomes clear it indicates the presence of phosphates.
·
No albumin is presence in the normal urine.
·
If the urine is highly acidic or highly alkaline, the reading
will be false.
Test for Acetone
·
Take 5 ml of urine in a test tube and saturate it with ammonium
sulphate.
·
Add a small crystal of sodium nitroprusside and mix well.
·
Slowly run along the side of the test tube liquor ammonia to
form a layer.
· Immediate formation of a purple permanganate colored ring at the junction of the two fluids indicates a positive test
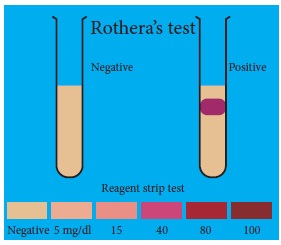
Glucose monitoring
Special points
·
Keep reagent tables in a cool, dry place at a temperature below
860 F(30o C).
·
Do not refrigerate the reagent tablets and strips.
·
Keep the container tightly closed.
·
Do not use discolored or outdated tablets or strips.
Test For Bile Salts (Hey’s Test)
·
Take a test tube, half full of urine.
·
Sprinkle sulphur powder on the surface of the urine.
·
If the powder sinks down to the test tube, it indicated the
presence of bile salts.
·
This is because, bile salts reduce the surface tension of the
urine and allows the sulphur powder to sink down.
Test for Bile Pigments
·
Fill three-fourth of a test tube with urine.
·
Add iodine drops along the sides of the test tube, so as to form
the layer on the surface of the urine.
·
A green color at the junction of the two liquids indicates the
presence of bile pigments.
·
Discard the urine and clean the test tube.
Related Topics