Chapter: Essential Anesthesia From Science to Practice : Clinical cases
Trauma patient under general anesthesia
Trauma patient under general
anesthesia
Learning
objectives:
·
anesthesia for the trauma patient
·
fluid management
·
increased intracranial pressure.
The
Emergency Department calls regarding an approximately 40-year-old man who was
thrown from his car during a traffic accident. He was briefly unconscious but
soon regained consciousness and was clearly intoxicated. Abdominal ultrasound
revealed a splenic injury; he has hematuria and multiple orthopedic injuries.
While in the CT scanner, the patient became more somnolent and now has a
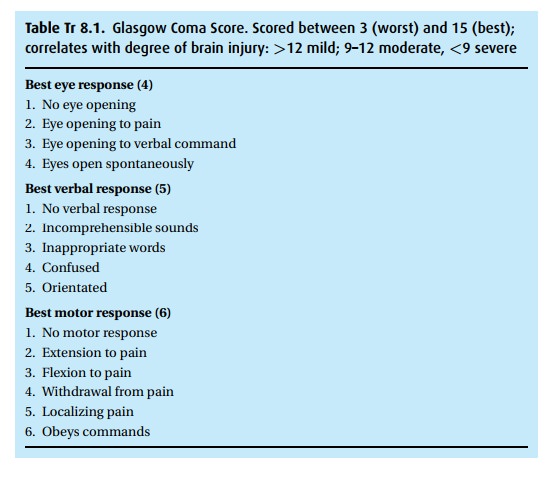
Glasgow Coma Score (GCS, Table Tr 8.1) of 9
(Eyes:2, Verbal:3, Motor:4). The scan was aborted, and the patient transported
directly to the trauma operating room. We alert all available staff to meet us
there, and confirm the blood bank is readying 8 units of type-specific blood
and 4 units of fresh frozen plasma.
This
case presents an acute emergency, with imminent risk to life (or limb). We have
little time for preoperative evaluation. In this patient, without family
around, we have no history, nor any information on medications or allergies. We
cannot obtain informed consent for the operation or anesthesia. In fact such a situation
mandates that we proceed in an attempt to save the patient’s life, even without
consent. We must evaluate his status as rapidly as possible, and induce
anesthesia such that the operation can begin.
Centers
designated to receive trauma cases maintain a “trauma operating room,” always
set up with the necessary equipment including rapid infusion sys-tems for warm
intravenous fluids, various vascular access devices, airway man-agement and
pressure monitoring equipment, and a selection of vasopressors.
We must rely on astute observation and physical examination. The GCS score tells us he has suffered at least a moderate brain injury. A full body survey might reveal tell-tale scars of past operations, for instance a sternotomy scar from a coronary artery bypass graft, or a small lower abdominal scar from an appen-dectomy. Bruises suggest locations of impact and elicit concerns over specific injuries. For example bruising over the ribs might indicate fracture and potential for pneumothorax and contusion of heart or lungs.
Physical
examination in the OR: Assessment of the ABCs (airway, breathing and
circulation) takes precedence. We find him breathing with good air movement,
reeking of alcohol, with a thready, rapid pulse. On more thorough examination
we find:
Caucasian
man of average build with obvious superficial trauma to face, chest, arms and
legs with numerous scrapes; hard cervical collar in place; weight ∼70 kg; height ∼6 (180 cm); bilateral chest tubes to water seal
BP 90/50
mmHg; HR 135 beats/min; respiratory rate 28 breaths/min
Airway:
patient uncooperative, difficult to fully assess; 4fb thyromental distance; in
hard cervical collar
CV: S1,
S2 no murmur, tachycardic
Respiratory:
Lungs clear to auscultation bilaterally
Neurologic:
somnolent, moving all extremities, withdraws to pain, pupils equal and
respon-sive to light
Access:
18 g intravenous catheter in right antecubital fossa, right subclavian
double-lumen catheter
This
patient has suffered multiple traumatic injuries. To get a handle on where we
stand we need to ask more questions of the surgeons, while simultaneously
applying monitors.
Further
history: He has an open left femur fracture; hematuria inferring kidney, ureter
or bladder injury; free fluid in the abdomen suggesting hemorrhage from spleen,
liver or intestines; multiple rib fractures but no evidence of pneumothorax; no
obvious cervical spine fracture on X-ray or CT; and a small right temporal
epidural hematoma on head CT. Chest tubes were placed on arrival in the
Emergency Department because of apparent rib fractures, subsequently a
subclavian catheter was inserted. He received a total of 4L Ringer’s lactate, 2
units Type O+, uncrossmatched blood and 3 mg i.v. morphine
in the Emergency Department.
Laboratories
and studies (from 30 min prior, before blood administered):
Hgb 9
g/dL; Hct 27%; Plt 150 000/µL;
Na 140
mEq/L; K 3.9 mEq/L; BUN 12 mg/dL; Cr 0.8 mg/dL; glucose 165 mg/dL (8.2 mmol/L)
PT and
aPTT: pending
Blood
type: A+ .
This additional history adds to our concern. The issues with which
we wrestle include the following:
·
Airway management We cannot rule out the presence of cervical
spine insta-bility or injury. Static radiographs cannot evaluate the quality of
the liga-ments that protect the cervical spinal cord from damage during head
move-ment as in traditional laryngoscopy. We consider all trauma patients to
have a full stomach, with risk of regurgitation and aspiration of gastric
contents. Standard application of cricoid pressure, a mainstay of aspiration
prophy-laxis, can displace a fractured cervical spine potentially compressing
the spinal cord. In the patient with spinal cervical injury we support the
poster-ior neck while compressing the cricoid ring, either with bi-manual
pressure or taking advantage of the posterior portion of the hard cervical
collar. Unfortu-nately that collar, with its bulk, proximity, and interference
with mouth opening, makes management of the airway difficult.
·
Intravascular volume status We find accurate assessment of volume status
dif-ficult. Significant blood can be lost into concealed spaces such as the
thigh and abdomen. If the abdomen is tense, the high pressure might curtail
intraabom-inal bleeding. Upon opening of the tight abdomen, a deluge of blood
might signal the release of the tamponade. Establishing appropriate vascular
access should be a high priority. In the presence of abdominal trauma, vascular
access must be sought in the upper body, as products administered through the
femoral route, for example, might be lost into the abdomen en route to the
central cir-culation. When the existing access is of inadequate caliber, as is
often the case, we can supplement it with additional catheters, or consider
exchanging one of the catheters over a wire (insert a long wire through the
catheter, remove the catheter, then advance a new, more appropriate catheter
over the wire). Fluid management should include consideration of hemoglobin
concentration, electrolytes, and osmolality (Ringer’s lactate is hypotonic).
Decreasing plasma osmolality contributes to brain swelling.
·
Pulmonary status Presence of rib fractures introduces the
likelihood of pneu-mothorax and/or pulmonary contusion. While not apparent on
an initial chest radiograph, decreasing pulmonary compliance with positive
pressure ventila-tion (increasing peak inspiratory pressure) could herald the
development of a pneumothorax, which should be noted and treated right away,
before becoming a tension pneumothorax.
·
Cardiovascular status With no knowledge of any pre-existing
cardiovasculardisease, we must focus on his current state. The hypotension and
tachycar-dia are most likely a function of his hypovolemia, but other causes
must be considered. High on the list would be cardiac tamponade or contusion,
ten-sion pneumothorax (if a chest tube is malfunctioning), fat embolism from
the femur fracture, transfusion reaction, anaphylaxis, spinal shock, and
electrolyte abnormalities (especially calcium from massive blood transfusion).
·
Neurologic status The fact the patient was conscious at the scene
gives rea-son to hope for a reasonable neurologic outcome, but his state is
becoming grave. With hypotension and likely increasing intracranial pressure
(ICP), we must concern ourselves with cerebral perfusion.1 The neurosurgeon will place an ICP
monitor, allowing calculation of the CPP. In the meantime, increas-ing blood
pressure takes precedence; we also consider measures to reduce the ICP
including hyperventilation, mannitol, avoiding a head-down position, e.g.,
Trendelenburg’s position, administering no hypotonic fluids and avoiding those
with glucose. Once a ventriculostomy has been placed, we can easily reduce the
CSF volume, and better monitor the actual CPP.
Preparation
for anesthesia. We talk to the patient reassuringly as we connect our standard
monitors and begin pre-oxygenation. We loosen his cervical collar sufficient to
view the trachea, while an assistant prepares the patient’s right wrist for a
radial arterial catheter.
In
trauma cases such as this we exercise our resource management skills and
encourage “parallel processing.” We orchestrate several helpers performing
simultaneous procedures, to facilitate a rapid beginning of the operation(s).
Despite
his altered mental status we continue to speak to the patient as we would want
our loved ones spoken to in a similar situation.
Induction
of anesthesia. Following adequate de-nitrogenation, we induce anesthesia with
etomidate 21 mg (∼0.3 mg/kg), fentanyl 100 mcg and succinylcholine 70 mg (∼1 mg/kg). One assistant provides in-line
stabilization of the spine without traction, and another applies bimanual
cricoid pressure, while we perform a gentle direct laryngoscopy and advance an
8.0 mm endotracheal tube through the vocal cords. After confirming the presence
of end-tidal CO2, we secure the tube and begin mechanical
ventilation with a rate of 15 breaths/min and a tidal volume of 600 mL,
titrated to an end-tidal CO2 of 25 mmHg.
We
prefer a rapid sequence induction because of aspiration risks, but find pros
and cons to all available agents. We wish to limit the systemic response to
intubation, reduce ICP, decrease the cerebral metabolic rate for oxygen (CMRO2),
while avoiding hypotension. In the presence of hypovolemia, cardiovascular
depression from thiopental and propofol can cause hypotension. Though often
considered the preferred agent in hypovolemia due to its stimulation of the
sym-pathetic nervous system, ketamine increases ICP and is therefore relatively
con-traindicated in this case. Etomidate usually causes little change in the
blood pres-sure, and reduces CMRO2, but can result in a hypertensive
response to intubation. For muscle relaxation we prefer succinylcholine for a
rapid-sequence induction, particularly when the airway examination is less than
optimal. Should intubation of the patient’s airway prove difficult, the
paralysis will last only a few minutes, then spontaneous respiration should
resume. Though succinylcholine can cause a small, transient increase in ICP, we
can blunt the effect with an adequate induc-tion agent and/or hyperventilation.
The non-depolarizing muscle relaxant alter-natives do not possess the rapid
onset and offset of succinylcholine, but become useful in patients at risk for
hyperkalemia (burns, crush injuries) or malignant hyperthermia.
We begin
hyperventilation after conferring with the neurosurgeon, who also requests
mannitol.
Induction
of anesthesia, continued. During the induction, an assistant placed a right
radial arterial catheter for continuous blood pressure measurement. While the
general surgeon prepares and drapes the abdomen, another assistant sterilely
places a 9-french “Swan Intro-ducer” catheter into the left subclavian vein.
All fluids are attached through warming cir-cuits. We draw blood for arterial
blood gas, electrolytes, hemoglobin and platelet concen-trations. We transduce
the arterial and central venous catheters: ABP 85/45 mmHg; HR 140 beats/min;
CVP 2 mmHg.
By
having an assistant place catheters, we free our hands for induction and
main-tenance of this critically ill patient. We choose the subclavian over
internal jugular route for vascular access to avoid any impairment to cerebral
venous drainage in this head-injured patient. The presence of chest tubes
reduces the risk of compli-cation from inadvertent pleural puncture. We send
blood for analysis of hema-tocrit to gauge the resuscitation and determine
needs for future blood products, as these take time to acquire from the blood bank.
Maintenance
of anesthesia. We maintain anesthesia with judicious administration of opi-oids
and isoflurane as tolerated in 50% inspired oxygen in air. We titrate the
oxygen con-centration to a saturation >95%, and the volatile agent to maintain hemodynamic
stability. Before the surgeon opens the abdomen, we administer a
non-depolarizing muscle relaxant and prepare for rapid infusion of fluids and
blood should the blood pressure suddenly fall.
For
abdominal operations we tend to avoid nitrous oxide for its propensity to
increase the volume of air-containing spaces. With vasopressors in hand and
ample vascular access, we are prepared for the abdomen to be opened
Intra-operative
event – Surgical incision. Upon opening the abdomen, the blood pressure falls precipitously
as several liters of blood are evacuated. We rapidly infuse normal saline and
begin infusing blood (already checked by nurses as to blood type and patient).
We ask the nurse to order more blood and fresh frozen plasma from the blood
bank. The Hemocue® ( -hemoglobin photometer) reads 7.2 g/dL. The surgeon
identifies a splenic rupture and successfully clamps the supplying artery. We
continue to administer blood based on the results of our laboratory and
Hemocue® evaluations.
Meanwhile
the neurosurgeon performs a small frontal craniotomy, draining about 75 mL
blood, then places an ICP monitor so that the CPP can be kept at 70–90 mmHg.
With the
bleeding apparently stopped and the hemodynamics stabilized at 110/60 mmHg with
a heart rate of 90 beats/min and a CVP of 8 mmHg, the surgeon closes the
abdomen to make room for the orthopedic surgeon to work on the femur fracture.
Suddenly the blood pressure plummets again.
Careful
evaluation of the findings can narrow the numerous potential causes for hypotension
in this setting. An increased central venous pressure might accompany cardiac
contusion, ischemia, tamponade, pulmonary embolism, or tension pneumothorax,
the latter associated with increased peak inspiratory pres-sures during
mechanical ventilation. Abdominal bleeding can be ruled out by direct
inspection. Continued hemorrhage concealed in the pelvis, retroperitoneal space
or thigh cannot be similarly ruled out, but should not cause such sudden
instability..
We place
a transesophageal echo (TEE) probe and find the right side of the heart
virtu-ally empty, and a fluid-density mass compressing the right ventricle. We
diagnose cardiac tamponade and the surgeon proceeds to insert a needle into the
pericardial sac, draining the pericardial blood with rapid improvement in
venous return as observed by TEE. But the hypotension does not resolve
completely, and the ventricle appears somewhat globally hypokinetic, we check
electrolyte levels and find a potassium of 4.5 mEq/L and an ionized calcium of
only 0.80 mmol/L (normal 1.03–1.30). The blood pressure responds to calcium
infusion.
The
femur fracture repair proceeds with much less fanfare.
When the
cause of hypotension remains unclear, transesophageal echocardiogra-phy might
prove helpful, as it did in this case. With massive transfusion, resulting
hypocalcemia can depress cardiac contractility, and electrolyte levels should
be assessed frequently. A word of warning, calcium drives potassium
intracellularly (part of its role in treating hyperkalemia); thus a patient
with hypokalemia can be pushed into ventricular fibrillation with rapid
infusion of calcium. The lesson – do not rapidly administer calcium without
first knowing the potassium level.
Emergence
from anesthesia. Following conclusion of the operation we leave the patient
paralyzed and sedated with his trachea intubated for transport to the Intensive
Care Unit. He will suffer major fluid shifts over the next few hours, with
possible pulmonary edema and airway swelling. Furthermore his neurologic status
is unclear. The sedative and paralytic drugs will be discontinued to allow
assessment of his neurologic status in the ICU.
Transport
of this patient requires manual ventilation with a Mapleson system and oxygen
source, and continuous monitoring. We bring along equipment to reintu-bate his
trachea, should that become necessary; we have at hand the vasoactive agents we
have required recently. In the ICU we give report, including updates on
laboratory values, to the nurse and physician. We remind them the replaced subclavian
catheter has not been radiographically evaluated, nor has the cervical spine
been medically cleared.
We
return to follow-up on the patient several times over the ensuing weeks.
Expected to make a full recovery eventually, he is discharged to a rehabilitation
center after three weeks.
N O T E
Cerebral
perfusion pressure (CPP) is calculated as mean arterial pressure minus ICP or
CVP, whichever is greater. We consider 60–80 mmHg an adequate CPP.
Related Topics