Chapter: Modern Analytical Chemistry: Introduction
The Analytical Perspective
The Analytical
Perspective
Having noted that each field
of chemistry brings
a unique perspective to the study of
chemistry, we now ask a second deceptively simple question. What is the “analyt-
ical perspective”? Many
analytical chemists describe this perspective as an analytical approach to solving problems.7 Although there are probably
as many descriptions of the analytical approach as there
are analytical chemists, it is convenient for our purposes to treat it as a five-step process:
·
Identify and define the problem.
·
Design the experimental procedure.
·
Conduct an experiment, and gather data.
·
Analyze the experimental data.
·
Propose a solution to the problem.
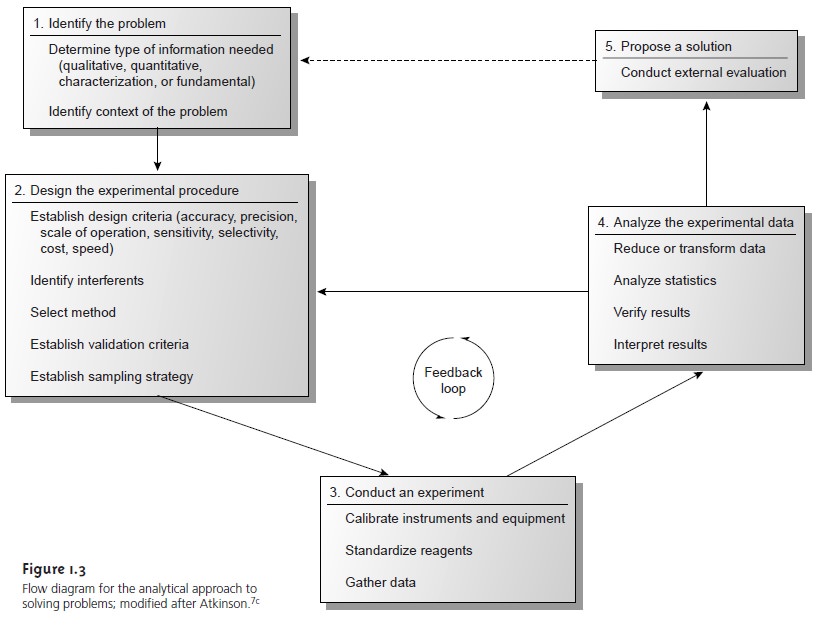
Figure 1.3 shows
an outline of the analytical approach along with some im- portant considerations at each step. Three
general features of this approach
de- serve attention. First, steps 1 and 5 provide opportunities for
analytical chemists to collaborate with individuals outside
the realm of analytical chemistry. In fact, many problems on which analytical chemists work originate in other fields.
Sec- ond, the analytical
approach is not linear, but incorporates a “feedback
loop” consisting of steps 2, 3, and 4, in which the outcome of one step may cause a
reevaluation of the other two steps. Finally,
the solution to one problem
often suggests a new problem.
Analytical chemistry begins
with a problem, examples of which include
evalu- ating the amount
of dust and soil ingested
by children as an indicator of environ- mental exposure
to particulate based pollutants, resolving
contradictory evidence
regarding the toxicity of perfluoro
polymers during combustion, or developing rapid and
sensitive detectors for
chemical warfare agents.* At this point
the analyti- cal approach
involves a collaboration between the analytical chemist and the indi-
viduals responsible for
the problem. Together
they decide what information is needed. It is also necessary for the analytical chemist to understand how the prob- lem
relates to broader
research goals. The type of information needed and the prob-
lem’s context are essential to designing an appropriate experimental procedure.
Designing an experimental procedure involves selecting an appropriate method of analysis based on established criteria, such as accuracy, precision, sensitivity, and detection limit; the urgency with which results are needed; the cost of a single analy- sis; the number of samples to be analyzed; and the amount of sample available for analysis.
Finding an appropriate balance between these
parameters is frequently complicated by their
interdependence. For example,
improving the precision of an analysis may require a larger sample.
Consideration is also given to collecting, stor- ing, and preparing samples,
and to whether chemical or physical interferences will affect the analysis. Finally, a good experimental procedure may still yield
useless in- formation if there is no method for validating the results.
The most visible
part of the
analytical approach occurs
in the laboratory. As part of the validation process,
appropriate chemical or physical standards
are used to calibrate any equipment being
used and any solutions whose
concentrations must be known.
The selected samples
are then analyzed
and the raw data recorded.
The
raw data collected during the experiment are then analyzed. Frequently the data must be reduced
or transformed to a more readily analyzable form. A statistical treatment of the data is used to evaluate
the accuracy and precision of the analysis and to validate the procedure. These
results are compared
with the criteria
estab- lished during the design of the experiment, and then the design is reconsidered, ad- ditional experimental trials are run, or a solution
to the problem is proposed. When a solution is proposed, the results are subject to an external
evaluation that may re-
sult in a new problem
and the beginning of a new analytical cycle.
As an exercise, let’s adapt this model of the analytical approach to a real prob- lem. For our example,
we will use the determination of the sources
of airborne pol- lutant particles. A description of the problem
can be found in the following article:
“Tracing Aerosol Pollutants with Rare Earth
Isotopes” by Ondov, J. M.; Kelly,
W. R. Anal. Chem.
1991, 63, 691A–697A.
Before continuing, take some time to read the article,
locating the discussions per- taining to each
of the five
steps outlined in Figure 1.3.
In addition, consider the fol- lowing questions:
·
What is the analytical problem?
·
What type of information is needed to solve the problem?
·
How
will the solution
to this problem be used?
·
What criteria were considered in designing the
experimental procedure?
·
Were there any potential interferences that had to be eliminated? If so, how were they treated?
·
Is
there a plan for validating the experimental method?
·
How
were the samples
collected?
·
Is
there evidence that
steps 2, 3, and 4 of the
analytical approach are
repeated more than once?
·
Was
there a successful conclusion to the problem?
According to our
model, the analytical approach begins with
a problem. The motivation for this research was to develop
a method for
monitoring the transport of solid aerosol particulates
following their release from a high-temperature com- bustion source. Because
these particulates contain significant concentrations of toxic heavy
metals and carcinogenic organic compounds, they represent a signifi-
cant environmental hazard.
An aerosol is a suspension of either a solid or a liquid
in a gas. Fog, for
exam- ple, is a suspension of small liquid water droplets
in air, and smoke is a suspension of small solid particulates in combustion gases.
In both cases
the liquid or solid par- ticulates must be small
enough to remain
suspended in the gas for an extended time. Solid aerosol particulates, which are the focus of this problem,
usually have micrometer or
submicrometer diameters. Over time, solid particulates settle out from the
gas, falling to the Earth’s
surface as dry
deposition.
Existing methods for
monitoring the transport of gases were
inadequate for studying aerosols.
To solve the problem, qualitative and quantitative information were needed
to determine the sources of pollutants and their net contribution to the
total dry deposition at a given
location. Eventually the
methods developed in this
study could be used to evaluate models
that estimate the
contributions of point sources of pollution to the level
of pollution at designated locations.
Following the movement
of airborne pollutants requires a natural
or artificial tracer (a species specific
to the source of the airborne pollutants) that can be exper-
imentally measured at sites distant
from the source.
Limitations placed on the tracer, therefore, governed the design of the experimental procedure. These limita- tions included cost, the need to detect small
quantities of the tracer, and the ab- sence of the tracer
from other natural
sources. In addition, aerosols are emitted from high-temperature combustion sources
that produce an abundance of very re- active species. The tracer,
therefore, had to be both
thermally and chemically stable. On the basis
of these criteria, rare earth isotopes, such as those
of Nd, were selected as tracers.
The choice of tracer, in turn, dictated
the analytical method
(thermal ionization mass spectrometry, or TIMS) for measuring the isotopic abundances of Nd in samples. Unfortunately, mass spectrometry is not a selective technique. A mass spectrum provides information about the
abundance of ions
with a given mass. It cannot distinguish, however, between different ions with the same mass. Consequently, the choice of TIMS
required developing a procedure for separating the tracer from the
aerosol particulates.
Validating the final experimental protocol
was accomplished by running a model
study in which 148Nd was released into the atmosphere from a 100-MW coal
utility boiler. Samples
were collected at 13 locations, all of which
were 20 km from
the source. Experimental results were
compared with predictions determined by the rate at which the tracer was released and the known
dispersion of the emissions.
Finally, the development of this procedure did not occur
in a single, linear pass through the analytical approach.
As research progressed, problems were encoun- tered and modifications made,
representing a cycle
through steps 2, 3, and 4 of the
analytical approach.
Others have pointed out, with
justification, that the analytical approach
out- lined here is not unique
to analytical chemistry, but is common
to any aspect of sci- ence involving analysis.8 Here, again, it helps
to distinguish between
a chemical analysis and analytical chemistry. For other analytically oriented scientists, such as
physical chemists and physical organic
chemists, the primary
emphasis is on the
problem, with the results of an analysis
supporting larger research
goals involving fundamental
studies of chemical or physical processes. The essence of analytical chemistry, however, is in the
second, third, and
fourth steps of the analytical ap- proach. Besides supporting broader research goals
by developing and validating an- alytical methods, these methods
also define the
type and quality
of information available to other research
scientists. In some cases, the success of an analytical method may even suggest
new research problems.
Related Topics