Chapter: Microbiology and Immunology: Antibodies
Structure of Immunoglobulins
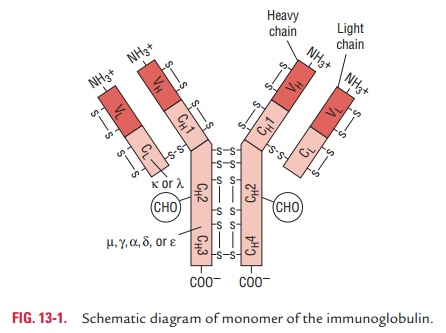
Immunoglobulins
There are five classes of immunoglobulins: (i) immunoglobulin G (IgG), (ii)
immunoglobulin M (IgM), (iii)
immunoglobulin A (IgA), (iv)
immunoglobulin E (IgE), and (v)
immunoglobulin D (IgD). Myeloma proteins were first used for the amino acid
sequencing of immunoglobulins. These proteins were also the first
immunoglobulins that were subjected to crystallographic studies. They provided
the first glimpses of the domain struc-ture of the prototypic immunoglobulin.
Structure of Immunoglobulins
Immunoglobulins show the following properties:
·
They are glycoproteins.
·
They are a complex structure of four polypeptide chains: two
identical heavy (typically 55 kDa each) chains and two identical light chains
(25 kDa each). This gives immuno-globulin an overall ‘Y’ or ‘T’ shape, which is
the most widely recognized feature of immunoglobulin structure.
The terms “heavy” and “light” refer to the molecular weights of the
chains. The heavy chains have a molecu-lar weight of 50,000–70,000 Da, while
light chains have a molecular weight of 25,000 Da. The heavy chains are longer,
and light chains are shorter (Fig. 13-1).
◗ Heavy chains
An immunoglobulin molecule has two heavy chains. Each heavy chain
is made up of 420–440 amino acids. The two heavy chains are held together by
one to five disulfide (S—S) bonds. Each heavy chain is bound to a light chain
by a disulfide bond and by noncovalent bonds, such as salt linkages, hydrogen
bonds, and hydrophobic bonds to form a heterodimer (H–L). Similar noncovalent
interactions and disulfide bridges link the two identical heavy and light (H–L)
chains to each other to form the basic four-chain (H–L)2 antibody
structure.
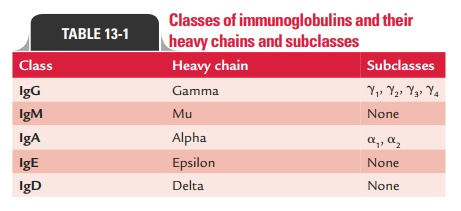
The heavy chains of a given
antibody molecule determine the class of that antibody. For example, IgM
contains mu ( ), IgG contains gamma ( ), IgA contains alpha ( ), IgD contains
delta ( ), and IgE contains epsilon ( ) heavy chains (Table 13-1). These heavy
chains are structurally and antigenically distinct for each class of
immunoglobulin. They differ in their size, car-bohydrate content, and as
antigens.
◗ Light chains
An immunoglobulin molecule has two light chains. Each light chain
is made up of 220–240 amino acids. Light chain is attached to the heavy chain
by a disulfide bond. The light chains are structurally and chemically similar
in all classes of immunoglobulins. They are of two types: kappa ( ) and lambda
( ). These two types differ in their amino acids present in constant regions.
Each immunoglobulin has either two or two chains but never both. The and chains
are present in human serum in a ratio of 2:1.
◗ Variable and constant regions
Each polypeptide chain of an immunoglobulin
molecule con-tains an amino terminal part and a carboxy terminal part. The
amino terminal part is called the variable region (V region) and the carboxy
terminal part is called the constant region (C region).
Both heavy and light chains contain variable and constant regions.
These regions are composed of three-dimensional folded structures with
repeating segments, which are called domains. Each heavy chain consists
of one variable (VH) and three constant (CH) domains. IgG and IgA have three CH
domains (CH1, CH2, and CH3), whereas IgM and IgE have four domains (CH1, CH2,
CH3, and CH4). Each light chain consists of one variable (VL) and one constant
domain (CL).
Variable region: The amino-terminal half of
the light or heavychain, consisting of 100–110 amino acids, is known as
vari-able or V regions (VL in light
chains and VH in heavy chains). V region is different for each class of
immunoglobulin.
The variable regions of both light and heavy chains consist of
three highly variable regions known as hypervariable regions. The antigen
combining sites Fab of the antibody molecule that consists of only 5–10 amino
acids each are present in the hypervariable region of both the light and heavy
chains. These antigen-binding sites are responsible for specific bind-ing of
antibodies with antigens. The high specificity of anti-bodies is primarily due
to the presence of these hypervariable regions.
Constant region: The carboxyl-terminal half of
the moleculeis called the constant (C) region. It consists of two basic amino
acid sequences. The Fc fragment, found to crystallize under low ionic
conditions, is present in the constant region of heavy chain.
The constant region of the heavy chain has many biological
functions. It is responsible for activation of the complement, binding to cell
surface receptors, placental transfer, and many other biological activities.
The constant region of the light chain has no biological function.
A single antibody molecule has two identical heavy chains and two
identical light chains, H2L2, or a multiple (H2L2)n of this basic four-chain structure. Subisotypes exist for and
chains, and this leads to the existence of subclasses of the respective
immunoglobulins.
Related Topics