Chapter: Microbiology and Immunology: Antibodies
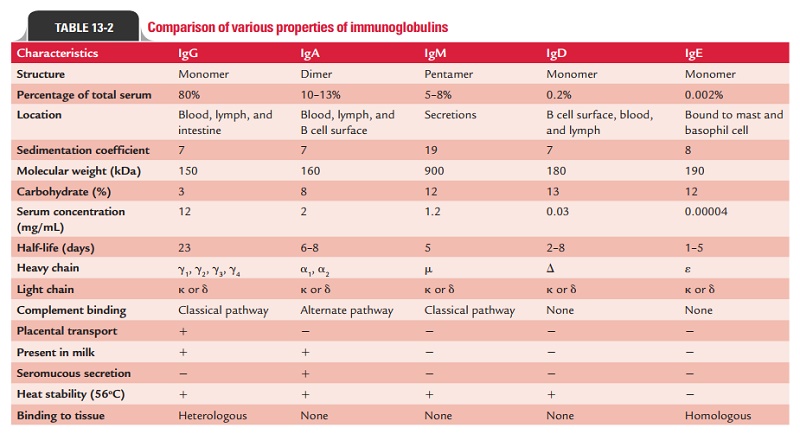
Immunoglobulin Classes
Immunoglobulin Classes
The structure and biological functions of five classes of
immu-noglobulins (IgG, IgM, IgA, IgE, and IgD) are described below:
◗ Immunoglobulin G
IgG is a 7S immunoglobulin with a molecular weight of 150,000 Da.
It has a half-life of 23 days—longest among all the immunoglobulins. Other
properties of the IgG are given in Table 13-2.
IgG is the most abundant class of immunoglobulins in the serum,
comprising about 80% of the total serum immuno-globulin. There are four IgG
subclasses IgG1, IgG2, IgG3, and IgG4—so numbered according to their decreasing
concentra-tions in serum. Though the differences between these subclasses are
minute, their functions vary as follows:
1.
IgG1, IgG3, and IgG4 are special because these are the only
immunoglobulins with the ability to cross the placen-tal barrier. They play an
important role in protecting the developing fetus against infections.
2.
IgG3, IgG1, and IgG2, in order of their efficiency, are effective
in the activation of the complement.
3.
IgG1 and IgG3 bind with high affinity to Fc receptors on phagocytic
cells and thus mediate opsonization. IgG4 has an intermediate affinity for Fc
receptors and IgG2 has an extremely low affinity.
Two chains, along with two or light chains, joined together by
disulfide bonds, comprise an IgG molecule as follows:
·
The γ chain is a 51-kDa, 450-amino acid residue heavy polypeptide chain.
·
It consists of one variable VH domain and a constant region with
three domains designated CH1, CH2, and CH3.
·
The hinge region is situated between CH1 and CH2.
·
Proteolytic enzymes, such as papain and pepsin, cleave an IgG
molecule in the hinge region to produce Fab and F (ab´) 2 and Fc fragments.
There are four subclasses of IgG in humans with four corre-sponding
γ chain isotypes designated
-1, -2, -3, and -4. IgG1, IgG2, IgG3, and IgG4 show differences in their hinge
regions and differ in the number and position of disulfide bonds that link two
chains in each IgG molecule. There is only a 5% difference in amino acid
sequence among human chain γ isotypes, exclusive of the hinge region. Cysteine resi-dues, which
make it possible for interheavy (γ ) chain disulfide bonds to form are found in
the hinge area. IgG1 and IgG4 have two interheavy chain disulfide bonds, IgG2
has 4, and IgG3 has 11. The IgG, is distributed equally in the intra- and extra-vascular
compartments.
◗
Immunoglobulin M
IgM constitutes about 5–8% of total serum immunoglobulins. It is
distributed mainly intravascularly. It is a heavy molecule (19S) with a
molecular weight varying from 900,000 to 1,000,000 Da (millionaire molecule). It
has a half-life of 5 days (Table 13-2).
IgM is basically a pentamer, composed of five immuno-globulin
subunits (monomeric subunits, IgMs) and one molecule of J chain. Each monomeric
IgM is composed of two light chains ( k or γ light chains) and two heavy chains ( μ). The heavy chains are
larger than those of IgG by about 20,000 Da, corresponding to an extra domain
on the constant region
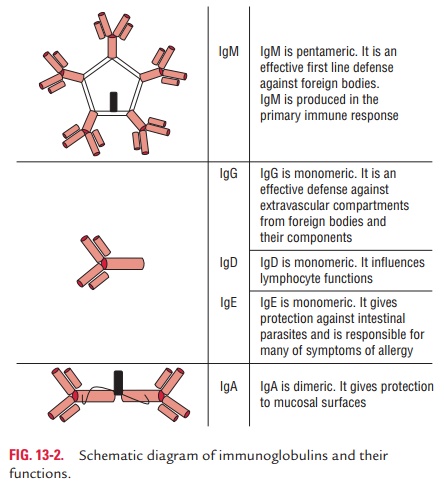
Two subclasses of IgM (IgM1 and IgM2) are described, which
differ in their chains. IgM1 consists of μ 1 and IgM2 consists of μ 2 chains (Fig. 13-2).
The immunoglobulin chain is a 72 kDa, 570-amino acid heavy
polypeptide chain comprising one variable region, des-ignated VH, and a
four-domain constant region, designated CH1, CH2, CH3, and CH4. The chain does
not have a hinge region. A “tail piece” is located at the carboxy terminal end
of the chain. It comprises 18-amino acid residues. A cysteine resi-due at the penultimate
position of a carboxy terminal region of the μ chain forms a disulfide bond that joins to the
J chain. There are five N-linked oligosaccharides in the μ chain of humans.
Monomeric IgM, with a molecular weight of 180,000 Da, is expressed
as membrane-bound antibody on B cells. As mentioned earlier, the J chain found
in the IgM molecule was believed to play a major role in the secretion of its
polymer-ized form. Being present on the membrane of B cells, IgM acts as the
antigen-binding molecule in the antigen–antibody complex.
Because of its pentameric structure with 10 antigen-binding sites,
serum IgM has a higher valency than the other isotypes. An IgM molecule can
bind 10 small hapten molecules; however, because of steric hindrance, only five
or fewer molecules of larger antigens can be bound simultaneously.
Treatment of serum with 2-mercaptoethanol destroys IgM without
affecting IgG antibodies. This forms the basis for differential estimation of
IgM and IgG antibodies in serum pre-treated with 2-mercaptoethanol.
◗ Immunoglobulin A
IgA is the second major serum immunoglobulin, comprising nearly
10–15% of serum immunoglobulin. It has a half-life of 6–8 days (Table 13-2).
IgA consist of heavy chain that confers class specificity on IgA
molecules. The chain is a 58-kDa, 470-amino acid residue heavy polypeptide
chain. The chain is divisible into three con-stant domains, designated CH1,
CH2, and CH3, and one vari-able domain, designated VH. Hinge region is situated
between CH1 and CH2 domains. An additional segment of 18-amino acid residues at
the penultimate position of the chain contains a cysteine residue where the J
chain can be attached through a disulfide bond. IgA occurs in two forms: serum
IgA and secre-tory IgA.
Serum IgA: It is present in the serum
and is a monomeric 7Smolecule with a molecular weight of 60,000 Da. It has a
half-life of 6–8 days. It has two subclasses, IgA1 and IgA2, which are two
-chain isotypes -1 and -2, respectively. The -2 chain has two allotypes, A2m
(1) and A2m (2), and does not have disulfide bonds linking heavy to light
chains. Differences in the two chains are found in two CH1 and five CH3
posi-tions. Thus, there are three varieties of -heavy chains in humans.
Secretory IgA: It is a dimer or tetramer and
consists of aJ-chain polypeptide and a polypeptide chain called secretory
component, or SC, or secretory piece (Fig. 13-3). The SC is a polypeptide with
a molecular weight of 70,000 Da and is pro-duced by epithelial cells of mucous
membranes. It consists of five immunoglobulin-like domains that bind to the Fc
region domains of the IgA dimer. This interaction is stabilized by a disulfide
bond between the fifth domain of the SC and one
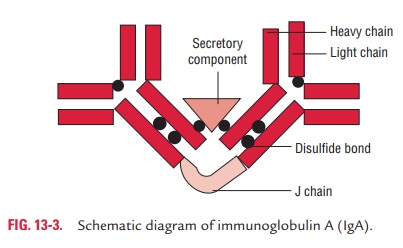
of the chains of the dimeric IgA. IgA-secreting plasma cells are
concentrated along mucous membrane surfaces. The daily production of secretory
IgA is greater than that of any other immunoglobulin. Secretory IgA is the
major immunoglobu-lin present in external secretions, such as breast milk,
saliva, tears, and mucus of the bronchial, genitourinary, and digestive tracts.
IgA activates the complement not by classical pathway but by alternative
pathway.
◗ Immunoglobulin E
IgE constitutes less than 1% of the total immunoglobu-lin pool. It
is present in serum in a very low concentration (0.3 g/mL). It is mostly found
extravascularly in lining of the respiratory and intestinal tracts. IgE is an
8S molecule with a molecular weight of 190,000 Da and half-life of 2–3 days.
Unlike other immunoglobulins that are heat stable, IgE is a heat-labile
protein—easily inactivated at 56°C in 1 hour (Table 13-2).
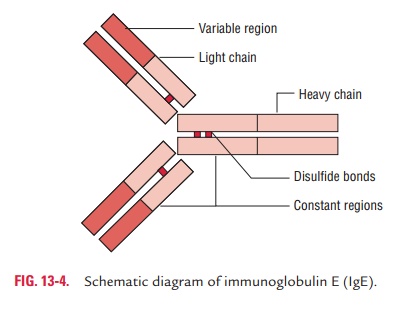
Two e heavy polypeptide chains, along with two or two light chains, fastened together by disulfide bonds, comprise an IgE molecule. The e chain is a 72-kDa, 550-amino acid residue
polypeptide chain. It consists of one variable region, designated VH, and a
four-domain constant region, designated CH1, CH2, CH3, and CH4. This heavy
chain does not possess a hinge region. In humans, the heavy chain has 428 amino
acid residues in the constant region (Fig. 13-4). IgE does not cross the
placenta or fix the complement.
◗ Immunoglobulin D
IgD comprises less than 1% of serum immunoglobulins. It is a 7S
monomer with a molecular weight of 180,000 Da. The half-life of IgD is only 2–3
days (Table 13-2). IgD has the basic four-chain monomeric structure with two
heavy chains (molecular weight 63,000 Da each) and either two or two light
chains (molecular weight 22,000 Da each) (Table 13-2).
Immunoglobulin chain is a 64-kDa, 500-amino acid residue heavy polypeptide chain consisting of one variable region, designated as VH, and a three-domain constant region, designated as CH1, CH2, and CH3. There is also a 58-residue amino acid residue hinge region in human chains. Two exons encode the hinge region. IgD is very susceptible to the action of proteolytic enzymes at its hinge region. Two separate exons encode the membrane component of chain. A distinct exon encodes the carboxy terminal portion of the human chain that is secreted. The human chain contains three N-linked oligosaccharides.
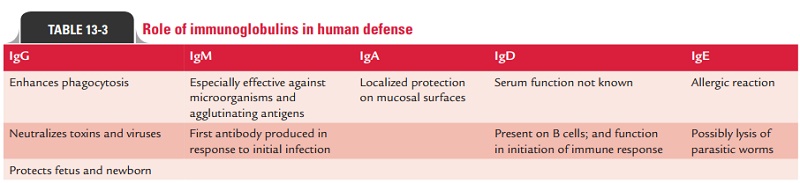
Table 13-3 summarizes roles of various immunoglobulins in human
defense.
Related Topics