Chapter: Civil : Construction Materials: Timber And Other Materials
Steel
Steel
Steel is the most suitable
building material among metallic materials. This is due to a wide range and
combination of physical and mechanical properties that steels can have. By
suitably controlling the carbon content, alloying elements and heat treatment,
a desired combination of hardness, ductility, and strength can be obtained in
steel. On the basis of carbon content steel may be classified as under:
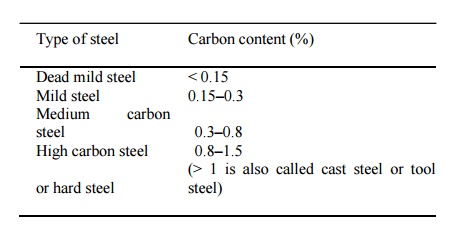
Type of steel Carbon content (%)
Dead
mild steel < 0.15
Mild
steel 0.15-0.3
Medium carbon steel 0.3-0.8
High
carbon steel or hard steel 0.8-1.5
(> 1 is also called cast steel or tool steel)
1 Manufacturing Methods
The
prominent steel-making processes are:
1. Bessemer process
2. Cementation process
3. Crucible process
4. Open Hearth process
5. Electric Smelting process
6. Duplex process
7. Lintz and Donawitz (L.D.) process
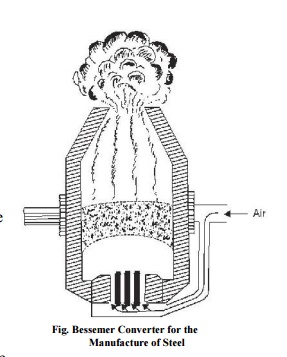
The most prominent
present-day steel-making process is the Bessemer process was introduced in
1856. The pig iron is first melted in Cupola furnace and sent to Bessemer
converter (Fig. ) Blast of hot air is given to oxidize the carbon. Depending
upon the requirement, some carbon and manganese is added to the converter and
hot air is blasted once again. Then the molten material is poured into moulds
to form ingots. L.D. process is modification of the Bessemer process in which
there in no control over temperature. By this method steel can be made in
hardly 25 minutes. In Open-hearth process also known as Siemen's-Martin
process, the steel produced is more homogeneous than by Bessemer's. The
electric process is costly but no ash or smoke is produced. The
Crucible process involves melting of blister steel or bars of wrought iron in
fire clay crucibles. Cast steel so obtained is very hard and is used for making
surgical equipments. The Duplex process is a combination of Acid Bessemer
process and Basic Open Hearth process
2 Properties and Uses
Mild Steel Also
known as low carbon or soft steel. It is ductile, malleable; tougher and more
elastic than wrought iron. Mild steel can be forged and welded, difficult to
temper and harden. It rusts quickly and can be permanently magnetized. The
properties are: Sp. gr. = 7.30, ultimate compressive and tensile strengths 800-1200N/mm2
and 600- 800N/mm2.
Mild steel is used in the form of
rolled sections, reinforcing bars, roof coverings and sheet piles and in
railway tracks.
High Carbon Steel: The
carbon content in high carbon steel varies from 0.55 to 1.50%. It is
also known as hard steel. It is tougher and more elastic than mild steel. It
can be forged and welded with difficulty. Its ultimate compressive and tensile
strengths are 1350 N/mm2 and 1400-2000 N/mm2,
respectively. Its Sp. gr. is 7.90.
High carbon steel is used for
reinforcing cement concrete and prestressed concrete members. It can take
shocks and vibrations and is used for making tools and machine parts.
High Tensile steel: The
carbon content in high tensile steel is 0.6-0.8%,
manganese 0.6%, silicon 0.2%, sulphur 0.05% and phosphorus 0.05%. It is
also known as high strength steel and is essentially a medium carbon steel. The
ultimate tensile strength is of the order of 2000 N/mm2 and a
minimum elongation of 10 per cent.High Tensile steel is used in prestressed
concrete construction.
3 Properties of Steel
The factors influencing the
properties of steel are chemical composition, heat treatment, and mechanical
work.
Chemical Composition
The presence of carbon in steel
gives high degree of hardness and strength. The addition of carbon to iron
decreases the malleability and ductility of the metal, and reduces its
permeability to magnetic forces.
The
tensile strength of hot rolled steel bars is maximum between 1.0 and 1.2 per
cent carbon. The elastic limit and the ultimate strength of steel increase with
carbon content but at a lower rate. The compressive strength of steel increases
directly with carbon content up to 1.0 per cent. The shear strength of steel
also increases with the carbon content. The ratio of shear strength to the
tensile strength is 0.80 for medium and low carbon steels and 0.60 for high
carbon steels. The modulus of elasticity is nearly same for tension and
compression and is practically independent of the carbon content.
The ductility of steel decreases
markedly as the carbon content increases. The resistance of steel to heavy
shocks or blows decreases with increase of carbon content.
Effects of Principal Impurities
on Steel: It is not feasible to entirely remove impurities in
making either iron or steel. The final product always contains small
percentages of the metallic impurities like silicon, manganese, sulphur, and
phosphorus besides iron and carbon. Occasionally small percentages of copper
and arsenic are also present. In well made steel these impurities generally
range between 0.2 and 1.0 per cent and their resultant effect on the
constitution of steel is often small. Of the common impurities, Phosphorus
cannot be eliminated in the process of manufacture, whereas most of the silicon
and manganese are introduced to improve the metal.
Silicon is often added to molten
metal to remove oxygen and diminish blow holes. In structural steel it rarely
exceeds 0.25 per cent. Silicon up to 1.75 per cent appears to increase both
ultimate strength and elastic limit without decreasing ductility.
Phosphorus is considered to
promote enlargement of the grains and thus produce brittleness. The ductility
of low-carbon steel decreases slightly by the presence of 0.3-0.5 per
cent phosphorus. However, yield point, ultimate strength and hardness of steel
are increased. Resistance to shock is also reduced by 0.1 per cent phosphorus
and the metal is rendered cold short (i.e., brittle when cold). A decrease in
toughness appears to be more pronounced in high-carbon than in low-carbon
steels. The maximum limits for phosphorus are: for inferior grades of
structural steel 0. 1, for best grades of structural steel 0.055, and 0.02 per
cent for tool steels.
Sulphur readily combines with
iron to form iron sulphide (FeS) which, when present in iron or steel, has a
tendency to segregate and form brittle networks at the grain boundaries. On
account of its low melting point, iron sulphide causes lack of cohesion between
adjacent grains when heated above a red heat. Such brittleness at high
temperature is termed as red shortness which makes steel or iron hard to roll
or forge. Manganese sulphide has a much higher melting point than iron
sulphidle and does not render ferrous metals red short. Therefore, inasmuch as
manganese has a very powerful affinity for sulphur, it is possible to relieve
red shortness by adding sufficient quantity of manganese to the molten metal to
combine with sulphur. Theoretically the ratio of manganese to sulphur should be
1.70 to 1.0 in order to form manganese sulphide and completely satisfy sulphur.
Less than 0.15 per cent sulphur content hardly exercises any appreciable effect
on the mechanical properties of steel. When sulphur is present along with
manganese it improves the machineability of steel.
Manganese has strong affinity for
oxygen and sulphur and acts as a cleanser of the molten metal by withdrawing
much of the undesirable impurities into the slag. Manganese increases the
tensile strength, hardenability and dilutes the effect of sulphur. When more
manganese is present than required for sulphur and oxygen the excess manganese
forms carbide and acts as hardener. Copper increases resistance to corrosion
when present in small percentage. Arsenic has a tendency to raise the strength
and brittleness. Non-metallic Impurities are mechanically suspended in the
metal and are often called slag inclusions causing brittleness.
Heat Treatment
The object of heat treatment is
to develop desired properties in steel. The properties of steel can be
controlled and changed as well by various heat treatments. A steel of given
composition may be made soft, ductile and tough by one heat treatment, and the
same steel may be made relatively hard and strong by another. Heat treatment
affects the nature, amount, and character of the metallographic properties.
Heat treatment influences the
solubility relations of the constituents, changes the crystallization either
with respect to form or degree of aggregation and introduces or relieves
internal stresses in the metal. The heat treatment process consists in
subjecting, a metal to definite temperature-time
course.
Some of
the principle purposes of heat treatment are as follows.
1. To
enhance properties such as strength, ductility, hardness and toughness.
2. To
relieve internal stresses and strains.
3. To refine
the grain.
4. To remove
gases.
5. To
normalize steel after heat treatment.
Hardening
This heat treatment consists of
heating the steel above the upper critical temperature holding at that
temperature until phase equilibrium has been established, and then quenching
rapidly to produce a martensite structure. Martensite is the chief constituent
of hardened steel and is fibrous or needle like structure. Hardened steel is
very brittle and cannot be used for practical purposes. The quenching medium is
usually brine, water or oil, depending on the desired cooling rate.
The objective of this treatment
may be to secure a given hardness to a desired depth in steel. But in most
instances the hardening treatment may simply be considered as starting point
from which better combinations of desired properties may be secured by
subsequent heat treatment. Fully hardened steel are not suitable for most
commercial uses because they are hard and brittle and have poor toughness.
Tempering
A plain
carbon steel that has been hardened is in metastable condition or equilibrium.
If this hardened steel is reheated to some temperature below the critical
range, a more stable condition will be obtained. Since hardened steels do not
usually have the combination of properties desired for specific uses,
modification is affected by tempering.
When a thick piece of steel is
cooled rapidly it develops additional strains as the surface cools quicker than
the interior. To relieve this strain, steel is subjected to the process
tempering which consists in slowly heating the steel to a predetermined
subcritical temperature and then cooling it slowly. This temperature varies
from 100 o C to 700 o C. The higher the temperature of tempering the softer is the
product. The properties like toughness and ductility are automatically
introduced with release of strain.
Annealing
It is a general term used for
heating and slow cooling of metal, glass or any other material, which has
developed strain due to rapid cooling.
The process consists of heating
the steel to a temperature below the critical range, but high enough to obtain
strain re-crystallization and then cooled in any manner. The exact heating
temperature depends on the composition of steel and the amount of work that it
has received, but is frequently between 500 o C to 600 o C. Annealing of steel in
addition to removing strain introduces one or more of the following properties.
1. Introduces
softness, ductility, and malleability.
2. Alters
electrical, magnetic, and other physical properties.
3. Produces
a definite microstructure and grain refinement.
4. Removes
gases.
Full annealing consists of
heating iron alloy 20 o C to 50 o C above critical temperature range, holding at
that temperature for the required period of time to convert it to austenite
followed by slow cooling. Full annealing usually decreases hardness, strength,
and resistance to abrasion, and increases ductility and merchantability.
Normalizing
It consists in heating steel
above critical range and cooling rapidly in air, but at rate slower than the
critical cooling rate. The purpose of this heat treatment is to refine the
grain structure resulting from rolling, forging or other manufacturing
processes.
Mechanical Works
Steel products are made by
casting molten refined steel of suitable composition into the desired form or
by mechanically working steel form the ignot through many intermediate forms to
the desired product. Mechanical work may be hot or cold. Mechanical working
involves many stages of hot working and may or may not include eventual cold
working.
The most important methods of hot
working steel are hot rolling, hammer forging, hydraulic and mechanical press
forging, and hot extrusion. Miscellaneous hot working methods include hot
spinning, hot deep drawing, hot flanging and hot bending, Heat treatment after
hot working is seldom used with low-carbon steels, whereas high-carbon steels
are always hardened and tempered.
The
principle methods of cold working steel are cold rolling, cold drawing and cold
extrusion. The cold working methods are used to provide increased strength,
accurate dimensions, and bright and scale free surfaces. Thin sheets and small
diameter wires are produced by cold-working methods. Cold working results in
increased density, hardness, and brittleness, and produces an internally
strained condition in the steel.
Mechanical work alters the form of the crystalline
aggregate and introduces internal stresses. Cold rolling increases the tensile
elastic limit from 15 to 97 per cent and tensile strength from 20 to 45 per
cent. In elastic resilience the cold-rolled metal is superior to the
hot-rolled, whereas in energy of rupture it is inferior to the hot-rolled
metal. The modulus of elasticity is slightly increased by cold rolling.
Practically, metals are rolled, forged, drawn, stamped and pressed.
Most of
steel building components-beams, rails, steels, bars,
reinforcement, pipes- are manufactured by rolling.
Rivets and bolts are made by forging operations. Thin-walled items (tubes) and
round, square, hexagonal rods of small cross-sectional areas (up to 10 mm2)
are manufactured by drawing. Stamping and pressing increases the buckling
strength of plates to be used for making them suitable for steel tanks and
containers. Steel trusses, towers, tanks, bridges and frames of multistorey
buildings are some of the examples of structures made of steel. The most common
and important application of steel in buildings is the rolled steel sections
and reinforcing bars and are described in the following sections.
Related Topics