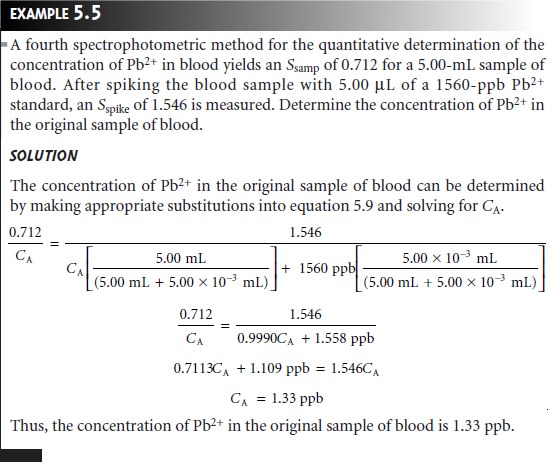
Chapter: Modern Analytical Chemistry: Calibrations, Standardizations, and Blank Corrections
Standardizing Methods: Standard Additions
Standard Additions
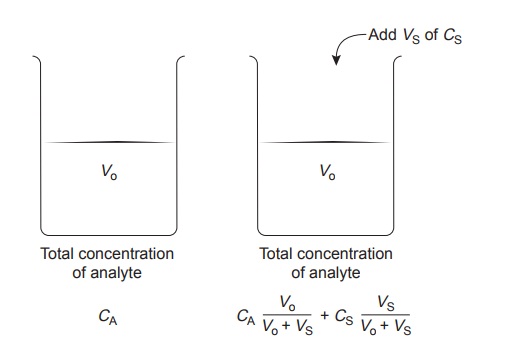
The complication of matching the matrix of the standards to that of the sample can be avoided by conducting the standardization in the sample. This is known as the method of standard additions.
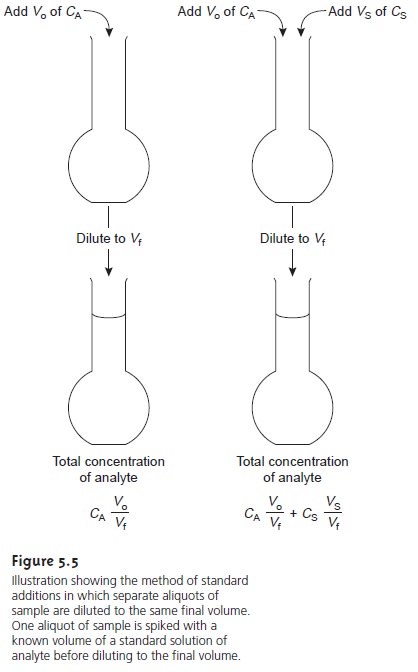
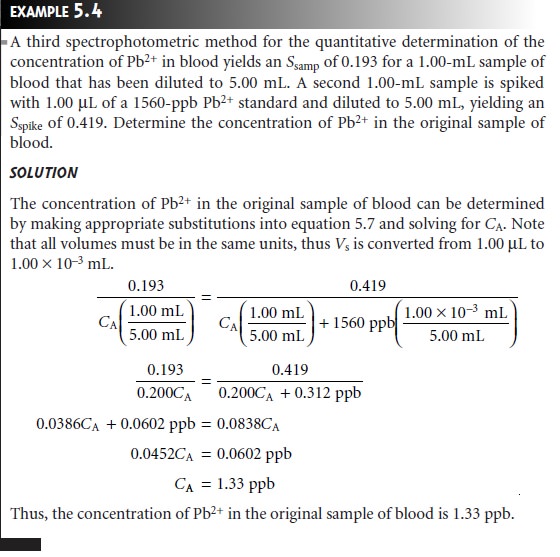
The simplest version of a standard addition is shown
in Figure 5.5. A volume,
Vo, of sample is diluted to a final
volume, Vf, and the signal,
Ssamp
is measured. A second identical
aliquot of sample is spiked with a volume,
Vs, of a standard
solution for which
the analyte’s concentration, CS, is known. The spiked sample
is diluted to the same final volume
and its signal, Sspike, is recorded. The following two equations relate
Ssamp and Sspike to the concentration of analyte, CA, in the original
sample
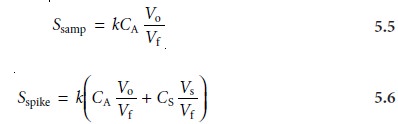
where the ratios
Vo/Vf and Vs/Vf account for
the dilution. As long as Vs is small rela- tive to Vo, the effect of adding the standard to the sample’s
matrix is insignificant, and the matrices of the sample
and the spiked
sample may be considered identical. Under these conditions the value of k is the same in equations 5.5 and 5.6. Solving
both equations for k and equating
gives
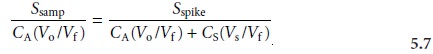
Equation 5.7 can be solved for the concentration of analyte in
the original sample.
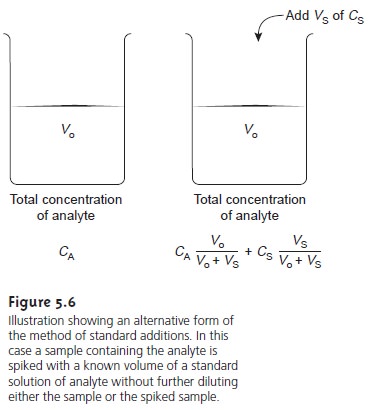
It also is possible to make a standard addition
directly to the sample after mea-
suring Ssamp (Figure 5.6).
In this case,
the final volume
after the standard addition is Vo + Vs and equations 5.5–5.7
become
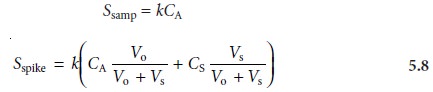
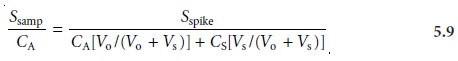
The single-point standard
additions outlined in Examples 5.4 and 5.5 are easily adapted to a multiple-point standard addition by preparing a series of spiked sam- ples
containing increasing amounts
of the standard. A calibration curve is prepared by plotting Sspike versus an appropriate measure of the amount of added standard. Figure 5.7 shows two examples of a standard
addition calibration curve based on equation 5.6. In Figure 5.7(a) Sspike is plotted versus the volume of the standard so- lution spikes, Vs. When k is constant, the calibration curve
is linear, and
it is easy to show that the x-intercept’s absolute
value is CAVo/CS.
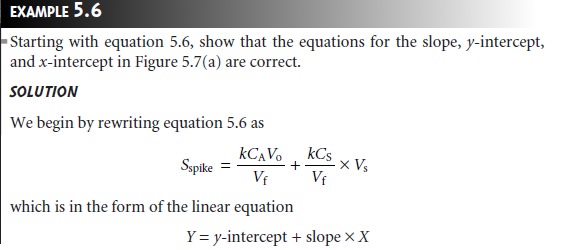
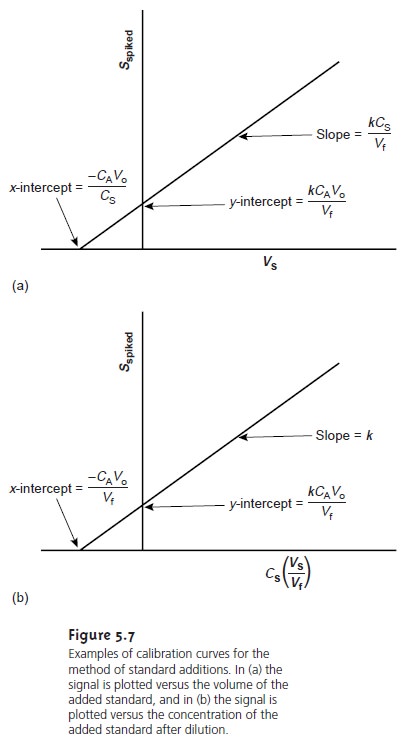
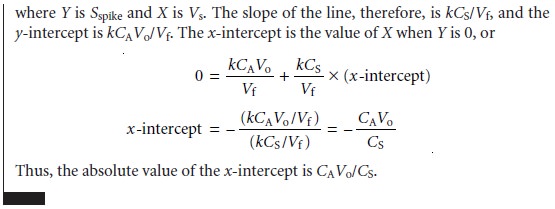
Since both Vo and CS are known, the x-intercept can be used to calculate the analyte’s concentration.
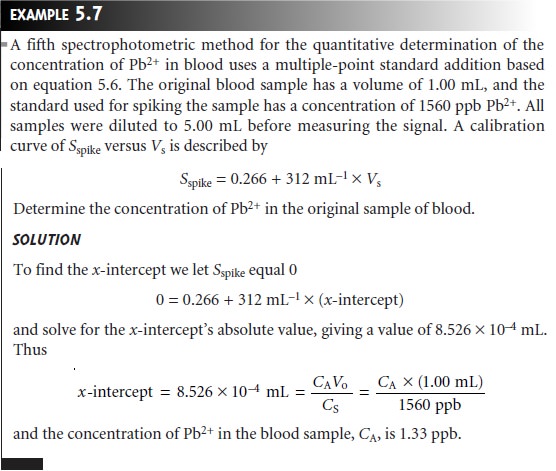
Figure 5.7(b) shows
the relevant relationships when Sspike is plotted versus
the con- centrations of the
spiked standards after dilution. Standard addition calibration curves based
on equation 5.8 are also possible.
Since a standard additions calibration curve
is constructed in the sample,
it cannot be extended
to the analysis of another
sample. Each sample,
therefore, re- quires its own standard
additions calibration curve.
This is a serious drawback
to the routine application of the method of standard
additions, particularly in labora-
tories that must handle many samples or that require
a quick turnaround time. For example, suppose
you need to analyze ten samples using a three-point calibration curve. For a normal calibration curve using external
standards, only 13 solutions
need to be analyzed (3 standards and
10 samples). Using
the method of standard
additions, however, requires
the analysis of 30 solutions, since each of the 10 sam-
ples must be analyzed three times (once before spiking
and two times after adding successive spikes).
The method of standard additions
can be used to check the validity
of an exter- nal standardization when matrix matching
is not feasible. To do this, a normal cali- bration curve of Sstand versus
CS is constructed, and
the value of k is determined from its slope. A standard additions calibration curve is then constructed using equation 5.6, plotting
the data as shown in Figure 5.7(b).
The slope of this standard additions calibration curve gives
an independent determination of k. If the two val- ues
of k are identical, then any difference between the sample’s matrix and that
of the external standards
can be ignored. When the values of k are different, a propor- tional determinate error is introduced if the normal
calibration curve is used.
Related Topics