Chapter: Modern Analytical Chemistry: Calibrations, Standardizations, and Blank Corrections
Standardizing Methods: Internal Standards
Internal Standards
The successful application of an external standardization or the
method of standard additions, depends
on the analyst’s ability to handle samples
and standards repro- ducibly. When a procedure cannot be controlled to the extent
that all samples
and standards are treated
equally, the accuracy and precision of the standardization may suffer. For example,
if an analyte is present
in a volatile solvent, its concentration
will increase if some solvent
is lost to evaporation. Suppose
that you have a sample and a standard with identical concentrations of analyte and identical signals.
If both experience the same loss of solvent
their concentrations of analyte and signals will continue to be identical. In effect, we can ignore
changes in concentration due to evaporation provided
that the samples
and standards experience an equivalent loss of
solvent. If an identical standard
and sample experience different losses of solvent,
however, their concentrations and signals will no longer be equal. In this
case, an external standardization or standard addition results in a determinate
error.
A standardization is still possible
if the analyte’s signal is referenced to a signal generated by another species
that has been
added at a fixed concentration to all samples and standards. The added species,
which must be different from the ana- lyte, is called an internal
standard.
Since the analyte
and internal standard
in any sample or standard
receive the same treatment, the ratio of their signals
will be unaffected by any lack
of repro- ducibility in the procedure. If a solution
contains an analyte
of concentration CA,
and an internal standard of concentration, CIS, then the signals
due to the analyte, SA, and the internal standard, SIS, are
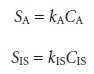
where kA and kIS are the sensitivities for the analyte
and internal standard,
respectively. Taking the ratio of the two signals gives
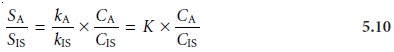
Because equation 5.10
is defined in terms of a ratio,
K, of the analyte’s sensitivity and the internal
standard’s sensitivity, it is not necessary to independently deter- mine values for either
kA or kIS.
In a single-point internal standardization, a single standard is
prepared, and K is determined by solving equation 5.10

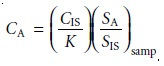
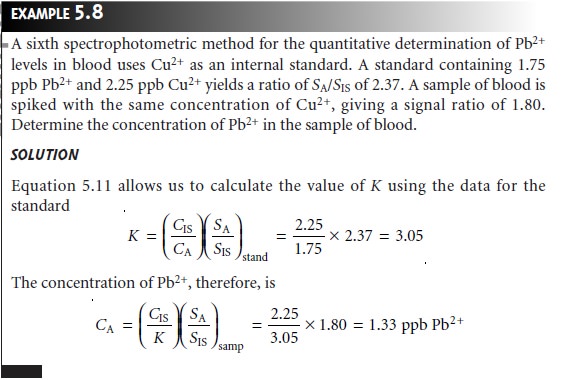
A single-point internal
standardization has the same limitations as a single-
point normal calibration. To construct an internal standard
calibration curve, it is
necessary to prepare several standards containing different concentrations of ana- lyte. These
standards are usually
prepared such that the internal
standard’s concen- tration is constant. Under
these conditions a calibration curve
of (SA/SIS)stand versus CA is linear with a slope of K/CIS.
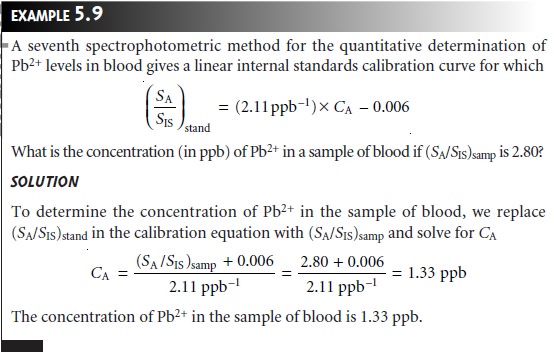
When the internal
standard’s concentration cannot
be held constant
the data must be
plotted as (SA/SIS)stand versus CA/CIS, giving a linear calibration curve with a slope
of K.
Related Topics