Chapter: Modern Analytical Chemistry: Calibrations, Standardizations, and Blank Corrections
Standardizing Methods: Single-Point versus Multiple-Point Standardizations
Single-Point versus Multiple-Point Standardizations*
The
simplest
way
to
determine the value of k in
equation
5.2
is
by
a single-point standardization. A single standard containing a known concentration of analyte, CS, is prepared and its signal,
Sstand, is measured. The value of k is calcu- lated as
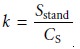 5.3
5.3
A single-point standardization is the least
desirable way to standardize
a method. When using a single standard, all experimental errors,
both de- terminate and indeterminate, are carried over into the calculated value for Any uncertainty in the value
of k increases the
uncertainty in the
ana- lyte’s concentration. In addition, equation
5.3 establishes the standardiza-
tion relationship for only a single concentration of analyte. Extending equation 5.3 to samples
containing concentrations of analyte different from that in the
standard assumes that
the value of k is constant, an as- sumption that is often
not true.
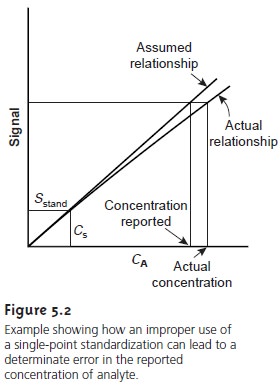
Figure 5.2 shows
how assuming a con-
stant value of k may lead to a determinate error. Despite these limitations,
single-point standardizations are
routinely used in many laboratories when the analyte’s range
of expected concentrations is limited. Under
these con- ditions it is often safe to assume that k is constant
(although this assump- tion should be verified
experimentally). This is the case,
for example, in clinical laboratories where many automated analyzers use only a single
standard.
The preferred approach
to standardizing a method is to prepare
a se- ries of standards, each containing the analyte at a different
concentration. Standards are chosen
such that they
bracket the expected range for the analyte’s concentration. Thus, a multiple-point standardization should
use at least three standards, although more are
preferable. A plot
of Sstand versus CS is known as a calibration curve. The exact standardization, or calibration relationship, is deter- mined by an appropriate curve-fitting algorithm.* Several
approaches to standard- ization are discussed in the following sections.
Related Topics