Chapter: Modern Analytical Chemistry: Calibrations, Standardizations, and Blank Corrections
Reagents Used as Standards

Reagents Used as Standards
The accuracy of a standardization depends on the quality of the reagents
and glass- ware used to prepare
standards. For example,
in an acid–base titration, the amount
of analyte is related to the absolute amount of titrant
used in the
analysis by the stoichiometry of the chemical reaction between the
analyte and the
titrant. The amount of titrant used
is the product
of the signal
(which is the
volume of titrant) and the titrant’s concentration. Thus, the accuracy
of a titrimetric analysis can be
no better than the accuracy
to which the titrant’s concentration is known.
Primary Reagents
Reagents used as standards are divided into primary reagents and secondary
reagents. A primary reagent
can be used to prepare
a standard con- taining an accurately known
amount of analyte.
For example, an accurately weighed sample of 0.1250 g K2Cr2O7 contains exactly 4.249 x 10–4 mol of K2Cr2O7. If this
same sample is placed in a 250-mL
volumetric flask and diluted to volume, the con-
centration of the
resulting solution is exactly 1.700
x 10–3
M. A primary
reagent must have a known stoichiometry, a known purity (or assay),
and be stable during
long-term storage both
in solid and
solution form. Because
of the difficulty in es- tablishing the
degree of hydration, even after drying,
hydrated materials usually
are not considered primary
reagents. Reagents not
meeting these criteria are called sec-
ondary reagents. The
purity of a secondary reagent
in solid form or the concentra-
tion of a standard prepared
from a secondary reagent must be determined relative to a primary
reagent. Lists of acceptable primary
reagents are available.4 Appendix 2 contains a selected listing
of primary standards.
Other Reagents
Preparing a standard often requires additional substances that are not
primary or secondary reagents. When a standard is prepared in solution, for
ex- ample, a suitable
solvent and solution
matrix must be used. Each of these
solvents and reagents is a potential source of additional analyte that, if unaccounted for, leads to a determinate error. If available, reagent grade chemicals conforming to standards set by the American Chemical Society should be
used. The
packaging label included with a reagent
grade chemical (Figure
5.1) lists either the maximum allowed limit for specific
impurities or provides
the actual assayed
values for the im-
purities as reported by the manufacturer. The purity of a reagent
grade chemical can be improved by purification or by conducting a more accurate assay. As dis- cussed later, contributions to Smeas from impurities in the sample
ma- trix can be compensated for
by including an appropriate blank
determination in the analytical procedure.

Preparing Standard Solutions
Solutions of primary
standards generally are
pre- pared in class A volumetric glassware to minimize
determinate errors. Even so, the relative error in preparing
a primary standard
is typically ±0.1%. The relative
error can be improved
if the glassware is first
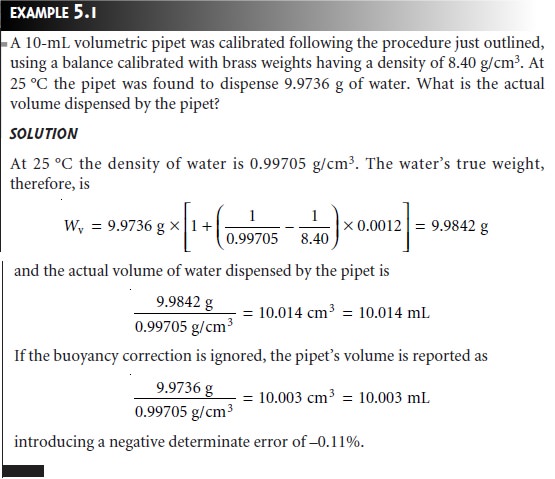
calibrated as described in Example 5.1. It
also is possible to prepare
standards gravimetrically by taking a known mass of stan- dard, dissolving it in a solvent,
and weighing the resulting solution.
Relative errors of ±0.01%
can typically be achieved in this fashion.
It is often
necessary to prepare
a series of standard solutions, each with a differ-
ent concentration of analyte. Such solutions may be prepared
in two ways. If the range of concentrations is limited to only one
or two orders
of magnitude, the
solu- tions are best prepared by transferring a known mass or volume
of the pure stan- dard to a volumetric flask and diluting
to volume. When working with larger con- centration ranges, particularly those extending over more than three orders of magnitude, standards are best prepared
by a serial dilution from a single
stock solution. In a serial
dilution a volume
of a concentrated stock solution, which is the first
standard, is diluted to prepare
a second standard. A portion of the second
standard is then diluted
to prepare a third standard, and the process
is repeated until
all nec- essary standards have been prepared. Serial dilutions must be prepared
with extra care because
a determinate error
in the preparation of any single standard
is passed on to all succeeding standards.
Related Topics