Chapter: 9th Science : Carbon and its Compounds
Special Features of Carbon
Special
Features of Carbon
The number of carbon
compounds known at present is more than 5 million. Many newer carbon compounds
are being isolated or prepared every day. Even though the abundance of carbon
is less, the number of carbon compounds alone is more than the number of
compounds of all the elements taken together. Why is it that this property is
seen in carbon and in no other elements? Because carbon has some unique
features such as:
·
Catenation
·
Tetra valency
·
Multiple bonds
·
Isomerism
·
Allotropy
1. Catenation
Catenation is binding
of an element to itself or with other elements through covalent bonds to
form open chain or closed chain compounds. Carbon is the most common element
which undergoes catenation and forms long chain compounds. Carbon atom links
repeatedly to itself through covalent bond to form linear chain, branched chain
or ring structure.
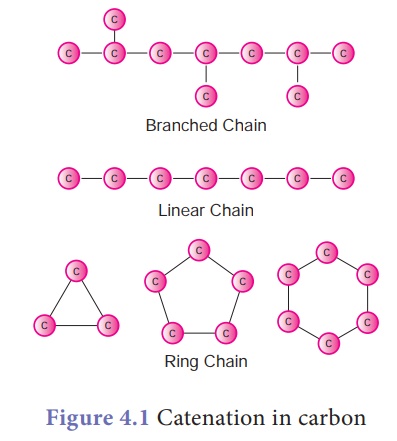
This property of carbon
itself is the reason for the presence of large number of organic carbon
compounds. So organic chemistry essentially deals with catenated carbon
compounds.
For example, Starch and
Cellulose contain chains of hundreds of carbon atoms. Even plastics what we use
in our daily life are macromolecules of catenated carbon compounds.
2. Tetravalency
Another versatile nature
of carbon is its tetravalency. The shell electronic configuration of carbon is
2,4 (Atomic no: 6). It has four electrons in its outermost orbit. According to
Octet Rule, carbon requires four electrons to attain nearest noble gas (Neon)
electronic configuration. So carbon has the tendency to share its four
electrons with other atoms to complete its octet. This is called its tetravalency.
Thus carbon can form four covalent bond with other elements.
For example, in methane,
carbon atom shares its four valence electrons with four hydrogen atoms to form
four covalent bonds and hence tetravalent.
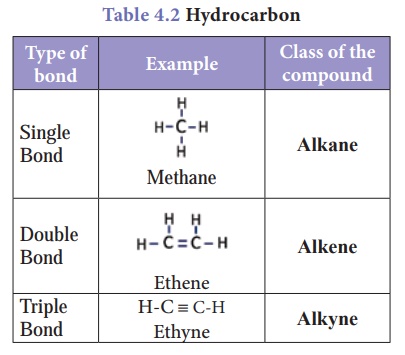
3. Multiple Bonds
As seen above, the
tetravalent carbon can form four covalent bonds. With this tetravalency, carbon
is able to combine with other elements or with itself through single bond,
double bond and triple bond. As we know, the nature of bonding in a
compound is the primary factor which determines the physical and chemical
characteristics of a compound. So the ability of carbon to form multiple bonds
is the main reason for the formation of various classes of carbon compounds.
Table 4.2 shows one of such classes of compounds called ‘hydrocarbons’
and the type of bonding in them.
When one or more
hydrogen in hydrocarbons is replaced by other elements like O, N, S, halogens,
etc., a variety of compounds having different functional groups are produced.
You will study about them in your higher class.
4. Isomerism
Isomerism is another
special feature of carbon compounds especially found in catenated organic
compounds. Let us consider the molecular formula of an organic compound C2H6O.
Can you name the compound? You can’t. Because the molecular formula of an
organic compound represents only the number of different atoms present in that
compound. It does not tell about the way in which the atoms are arranged and
hence its structure. Without knowing the structure, we can’t name it.
A given molecular
formula may lead to more than one arrangement of atoms. Such compounds are
having different physical and chemical properties. This phenomenon in which the
same molecular formula may exhibit different structural arrangement is
called isomerism. Compounds that have the same molecular formula but
different structural formula are called isomers (Greek, isos = equal, meros =
parts).
Illustration:
The given formula C2H6O
is having two kinds of arrangement of atoms as shown below.
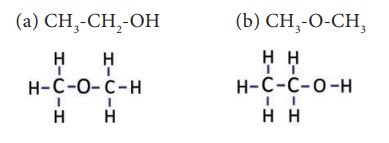
Both the compounds have
same molecular formula but different kind of arrangements. In compound ‘a’, the
oxygen atom is attached to a hydrogen and a carbon. It is an alcohol. Whereas
in compound ‘b’, the oxygen atom is attached to two carbon atoms and it is an ether.
These compounds have different physical and chemical properties. You will study
about isomerism in detail in higher classes.
5. Allotropy
Allotropy is a property by which
an element can exist in more than one form that are physically different
and chemically similar. The different forms of that element are called its
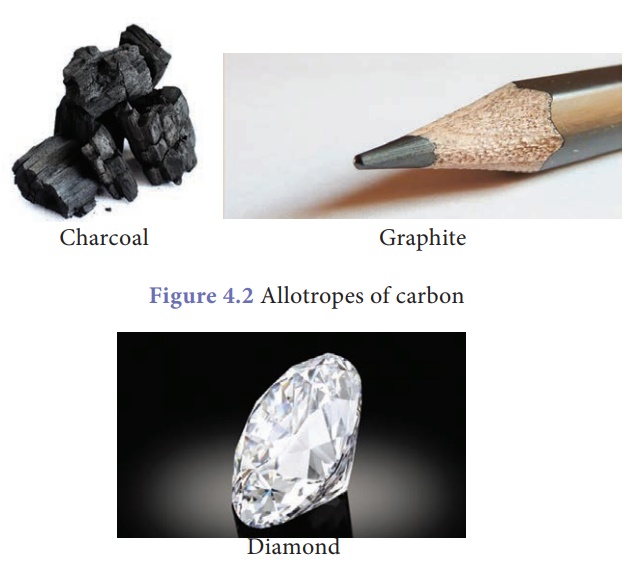
allotropes. Look at the materials given below. They are charcoal, graphite and
diamond.
Are they equally hard?
Are they cost same? Definitely not. Diamond is shiny, costliest and hardest of
all. Charcoal and graphite are soft and dark. But chemically they are all
similar. Yes. They are made of only carbon. They are called allotropes of
carbon.
Why do elements show allotropy?
The main reason for the
existence of allotropes of an element is its method of formation or preparation.
Carbon exists in
different allotropic forms and based on their physical nature they are
classified as below.
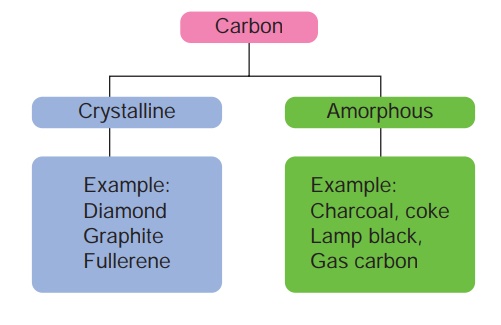
(a) Crystalline forms of Carbon
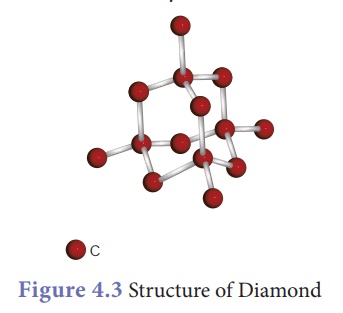
Diamond:
·
In diamond, each carbon atom shares its four valence
electrons with four other carbon atoms forming four covalent bonds.
·
Here the atoms are arranged in repeated tetrahedral fashion which leads to a
three dimensional structure accounting for its hardness and
rigidity.
Graphite:
·
In graphite, each carbon atom is bonded to three other carbon atoms
through covalent bonds in the same plane.
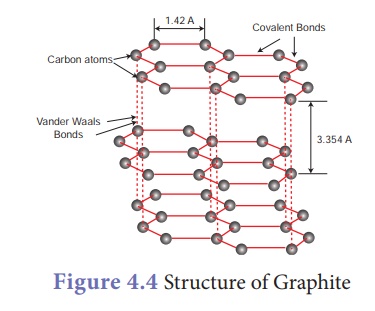
·
This arrangement forms hexagonal layers which are held together
one over other by weak Vander Waals forces.
·
Since the layers are held by weak forces, graphite is softer than
diamond.
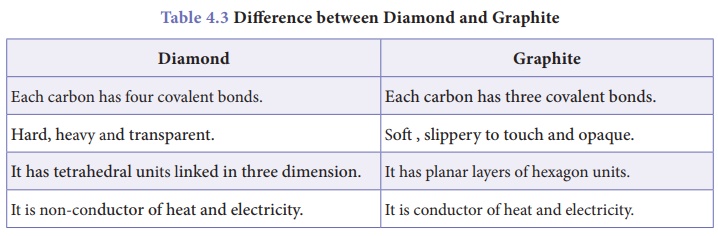
Difference between Diamond and Graphite
Diamond
·
Each carbon has four covalent bonds.
·
Hard, heavy and transparent.
·
It has tetrahedral units linked in three dimension.
·
It is non-conductor of heat and electricity.
Graphite
·
Each carbon has three covalent bonds.
·
Soft , slippery to touch and opaque.
·
It has planar layers of hexagon units.
·
It is conductor of heat and electricity.
Fullerene:
The third crystalline
allotrope of carbon is fullerene. The best known fullerene is Buckminster
fullerene, which consists of 60 carbon atoms joined together in a
series of 5- and 6- membered to form spherical molecule resembling a soccer
ball. So its formula is C60.
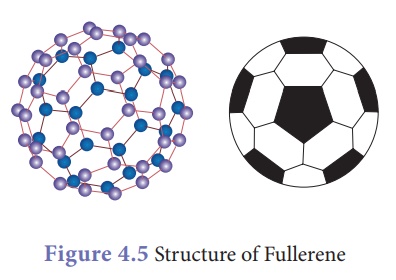
This allotrope was named
as Buckminster fullerene after the American
architect Buckminster fuller. Because its structure remembered
the framework of dome shaped halls designed by Fuller for large
international exhibitions, it is called by the pet name Bucky Ball.
A large family of fullerenes exists, starting at C20 and
reaching up to C540.
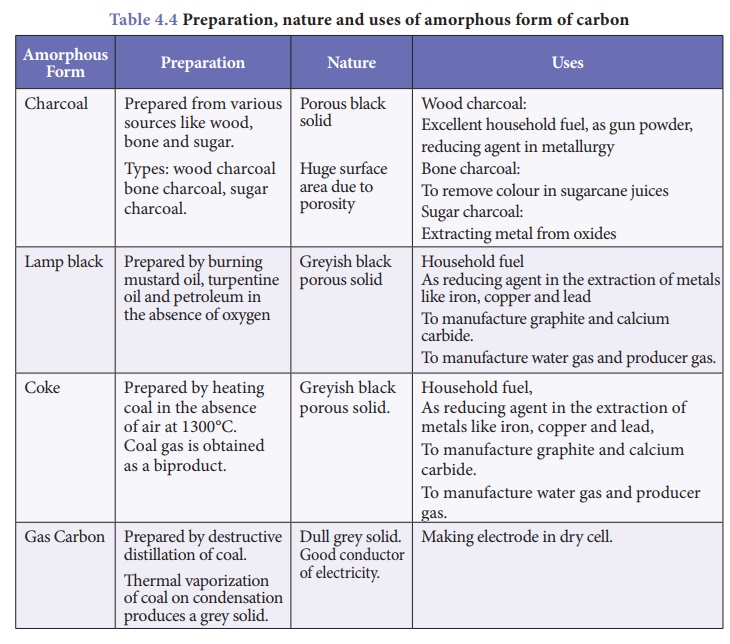
(b) Amorphous forms of carbon
In amorphous form of
carbon, carbon atoms are arranged in random manner. These form of carbon are
obtained when wood is heated in the absence of air. Table 4.4 enlists some
amorphous forms of carbon and their features.
Related Topics