Chapter: Civil : Environmental Engineering : Sewage Treatment
Sewage Treatment : The Operation
Sewage
Treatment : The Operation
Denitrification occurs
when oxygen levels are depleted and nitrate becomes the primary oxygen source
for microorganisms.
The process is
performed under anoxic conditions, when the dissolved oxygen concentration is
less than 0.5 mg/L, ideally less than 0.2.
When bacteria break
apart nitrate (NO3-) to gain the oxygen (O2), the nitrate is reduced to nitrous
oxide (N2 O), and, in turn, nitrogen gas (N2).
For the process to
proceed, the bacteria needs a carbon source. This can be obtained from carbon
within the waste or a small amount of primary effluent can be added.
Alternatively, an external source of carbon can be provided (Methanol).
After leaving the
anoxic tank, the wastewater is aerated for
10 to 15 minutes to
drive off the Nitrogen gas and add oxygen to the wastewater before
sedimentation
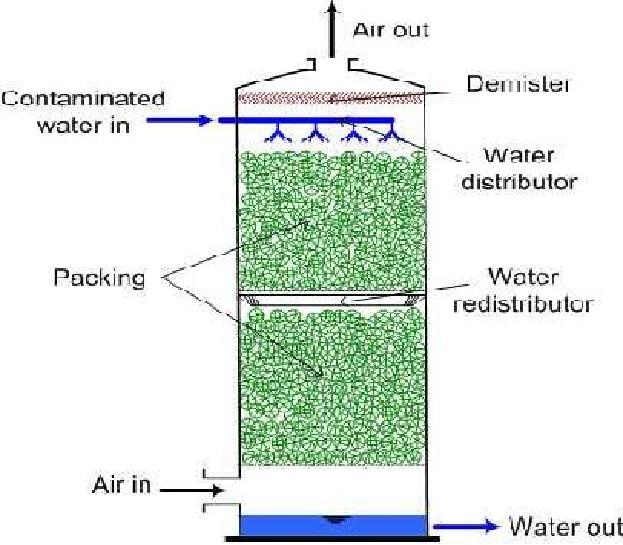
The Air Stripping Process
The process consist of
converting the amm liquid in air
The gaseous phasehase NH3NH4+ exist and together the
inequilibrium aqueousand the p dominance of any one is dependent on pH and
Temperature. A pH of >11 is required for complete conversion to NH3
The Operation
Lime is
used to raise
the pH to
>11
Stripping-gasificationofismostdeefficiently
done using a counter current spray tower.
Design Parameters are:
2000-6000m3 of air / m3
wastewater
Tower Depths
> 7.5 m
HRL- 4640L/min/m2 of tower
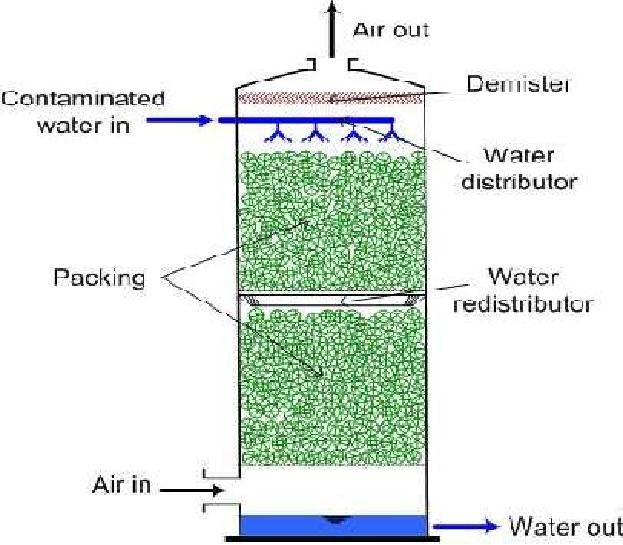
Advantages and Disadvantages of the air
Stripping
Air strippingemosteconomicalismeans
thofremoving nitrogen, however, as temperature approaches freezing the
efficiency drops significantly.
Noise pollution
by roaring fans.
Air pollution
by odor caused by release
of
Additionimecause
softeninglof WW of alkalinity.
The precipitation of
calcium carbonate on the packed media therefore requires continuous cleaning.
Related Topics