Chapter: Modern Analytical Chemistry: Kinetic Methods of Analysis
Radiochemical Methods of Analysis: Characterization Applications
Characterization Applications
One example of a characterization application is the
determination of a sample’s
age based on the kinetics for the decay
of a radioactive isotope present
in the sample.
The most common
example is carbon-14 dating, which is used to determine the age
of natural organic
materials.
|
7 |
The 146C then migrates into the lower atmosphere, where it is oxidized to produce radioactive CO2, which is subsequently incorporated into living organisms. As a result,
all living plants and
animals have approximately the same
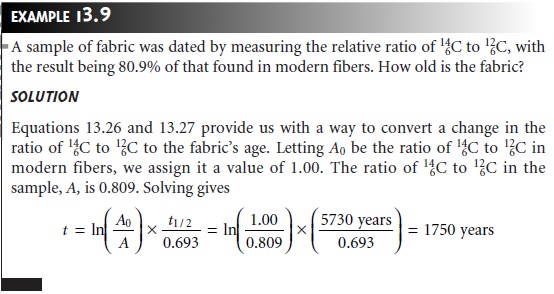
ratio of 146C to 126C in their tissues. When an organism
dies, however, the radioactive decay of 146C to 147N by β-emission (t1/2 = 5730 years) results in a gradual reduction in the
ratio of 146C to 147C. The change
in this ratio can be used to date samples that are as much as 30,000 years old, although
the precision of the analysis
is best when the sample’s
age is less than 7000 years.
The accuracy of carbon-14 data is limited
by the assumption that the ratio of 146C to 126C has remained constant
over time. Some variation in the ratio has
occurred as the result of the increased consumption of fossil fuel, and the
production of 146C during the testing of nuclear weapons.
Correction factors have been de- veloped that increase the
accuracy of carbon-14 dating
Other isotopes can be used
to determine the
age of samples. The age of rocks,
for example, has been determined from the ratio
of the number
of radioactive 23892U atoms to the number of stable 20682Pb atoms produced
by radioactive decay. For rocks
that do not contain uranium,
dating is accomplished by comparing the ratio
of radioactive 4019K to the stable
4018Ar. A nother example is the dating of
sediments col- lected from lakes by measuring the amount of 21082Pb
present.
Related Topics