Chapter: Modern Analytical Chemistry: Kinetic Methods of Analysis
Radiochemical Methods of Analysis: Quantitative Applications
Quantitative Applications
Three common quantitative applications of radiochemical methods of analysis
are considered in this section: the direct analysis
of radioactive isotopes
by measuring their rate of disintegration, neutron activation, and the use of radioactive isotopes as tracers in isotope dilution.
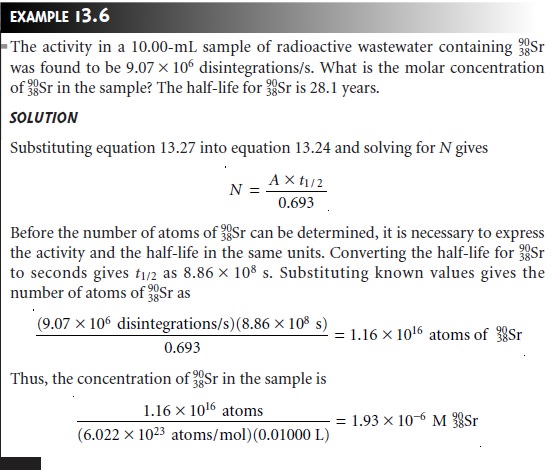
Direct Analysis of Radioactive Analytes
The concentration of a long-lived ra- dioactive isotope
is essentially constant
during the period of analysis.
As shown in Example 13.6, the sample’s
activity can be used to calculate the number of radioac-
tive particles that are present.
The direct analysis
of short-lived radioactive isotopes using the method out- lined in Example 13.6
is less useful
since it provides only a transient measure of the isotope’s concentration. The
concentration of the isotope at a particular
moment can be determined by measuring its
activity after an elapsed time,
t, and using equa- tion 13.26 to calculate
N0.
Neutron Activation Analysis
Few samples
of interest are naturally radioactive. For many elements, however, radioactivity may be induced by
irradiating the sample with neutrons in a process called neutron activation
analysis (NAA). The radioac- tive element
formed by neutron activation decays to a stable isotope
by emitting gamma rays and, if necessary, other
nuclear particles. The rate of gamma-ray emis- sion is proportional to the analyte’s
initial concentration in the sample.
For exam- ple, when a sample
containing nonradioactive 2713Al is placed in a nuclear
reactor and irradiated with
neutrons, the following nuclear reaction results.
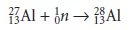
The radioactive isotope
of 2713Al has a characteristic decay process that includes the release of a beta particle and a gamma ray.
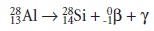
When irradiation is complete, the sample is removed from the nuclear
reactor, al- lowed to cool while
any short-lived interferences that might be present decay
to the background, and the rate of gamma-ray emission is measured.
The initial activity at the end of irradiation depends on the
number of 2813Al atoms that are
present. This, in turn, is equal to the difference between the rate
of formation for 2813Al and its rate of disintegration,
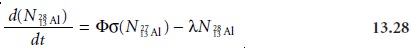
Where Φ
is the neutron
flux, and is
the reaction cross-section, or probability for the
capture of a neutron by the 2713Al nucleus.
Integrating equation 13.28
over the time of irradiation, ti, and multiplying
by gives
the initial activity, A0, at the end of
irradiation as
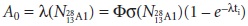
|
13 |
A simpler approach
for analyzing neutron
activation data is to use one or more
external standards. Letting
(A0)x and
(A0)s represent the initial activity
for the ana- lyte in an unknown and a single external standard,
and letting wx and
ws represent the weight of analyte
in the unknown and the external standard,
gives a pair of equations
(A0)x = kwx ……………….13.29
(A0)s = kws ……………….13.29
that can be solved to determine the mass of analyte in the
sample.
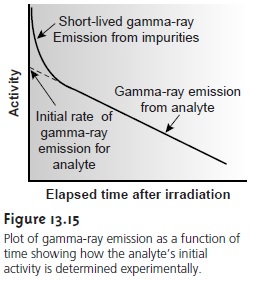
As noted earlier,
gamma-ray emission is measured following a “cooling” period in which short-lived interferents are allowed to decay away.
The initial activity therefore, is determined by extrapolating a curve of activity versus
time back to t =
0 (Figure 13.15).
Alternatively, if the
samples and standards are irradiated simul- taneously, and the activities are measured at the same
time, then these
activities may be used
in place of (A0)x and (A0)s in the preceding equations.
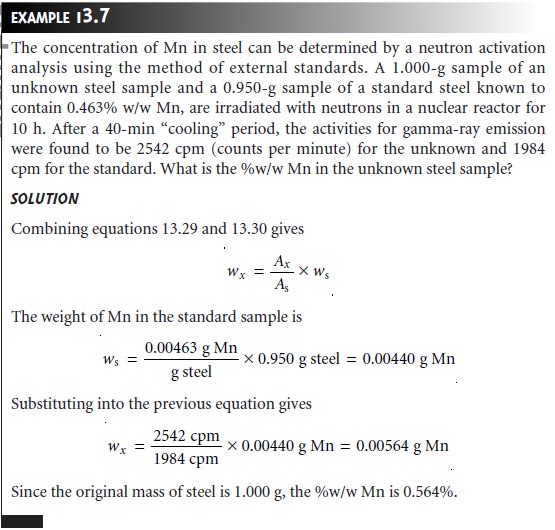
One of the important advantages of NAA is its applicability to almost all ele-
ments in the periodic table.
Another advantage of neutron activation is that it is
nondestructive. Consequently, NAA
is an important technique for
analyzing ar- chaeological and forensic samples,
as well as works of art.
Isotope Dilution
Another important quantitative radiochemical method is isotope
dilution. In this method of analysis a sample of analyte, called
a tracer, is prepared in a radioactive form
with a known
activity, AT, for its radioactive decay. A mea- sured mass of the
tracer, wT, is added
to a sample containing an unknown mass,
wx, of a nonradioactive analyte, and the material is homogenized. The
sample is then processed to isolate wA grams of purified
analyte, containing both radioactive and nonradioactive materials. The activity
of the isolated sample, AA, is measured. If all
the analyte, both radioactive and nonradioactive, is recovered, then AA and AT will be equal. Normally,
some of the analyte is lost during isolation and purification. In this
case AA is less than AT, and
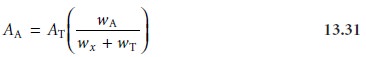
The ratio of weights in equation 13.31
accounts for the “dilution” of the activity
due to a failure
to recover all
the analyte. Solving
equation 13.31 for wx gives

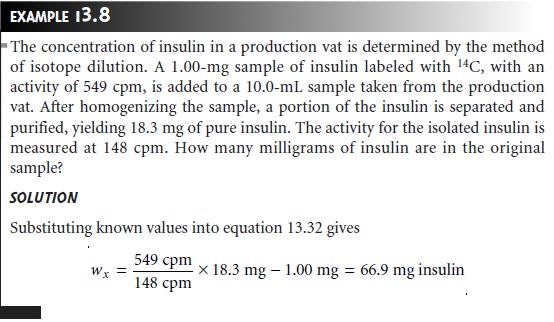
Equations 13.31 and 13.32 are only valid
if the radioactive element in the tracer
has a half-life that is considerably longer than the time needed to conduct
the analysis. If this is not the case,
then the decrease
in activity is due both to the effect of dilu-
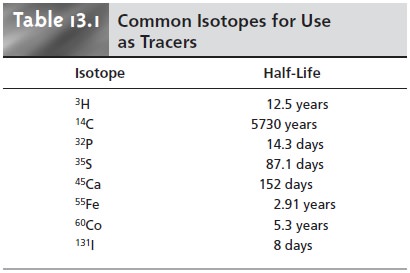
tion and the natural decrease in the isotope’s activity. Some common
radioactive isotopes for use in isotope
dilution are listed
in Table 13.1.
An important feature
of isotope dilution
is that it is not necessary to recover all the
analyte to determine
the amount of analyte present
in the original sample. Iso- tope
dilution, therefore, is useful for
the analysis of samples with
complex matrices, when a complete recovery
of the analyte is difficult.
Related Topics